Thoracic Imaging of the Pediatric Intensive Care Patient 6 Normal Thoracic Findings in Newborns Catheter Position: Normal Findings and Malposition Transient Tachypnea of the Newborn (Wet Lung) Infantile Respiratory Distress Syndrome Complications during or after Mechanical Ventilation Congenital Lung Diseases with Respiratory Failure at Birth Acute Obstruction of the Upper Airways Acute respiratory failure in children may be due to a variety of causes, depending on the age of the patient. Accordingly, an accurate knowledge of patient age is a key factor in the interpretation of radiologic findings. This chapter deals solely with acquired acute diseases and congenital thoracic diseases that may necessitate treatment in an intensive care unit. Issues relating to congenital heart disease may be found in the specialized literature. The imaging modality of choice is the chest radiograph, usually limited to the anteroposterior (AP) projection, and it may be supplemented by ultrasonography. Computed tomography (CT) (with age-adapted protocols) is reserved for cases with complications and equivocal radiographic findings. The correct interpretation of thoracic findings in newborns requires an accurate knowledge of the patient’s age at the time of the imaging study. It is also essential to know the timeline of normal radiologic findings. This applies particularly to the interpretation of chest radiographs during the first days of life. Moreover, there are several pitfalls that may cause difficulties for the less experienced examiner (Table 6.1). Fetal lung fluid. Normally, the first new postnatal breaths leads to good aeration of both lungs, and it may take several hours for the fetal lung fluid to be completely absorbed from the alveoli. This absorption takes place through the pulmonary interstitium and lymphatic vessels. It is marked by an increasing radiographic lucency which starts at the center of the lungs and spreads to the upper lobes and then to the lower lobes. Fetal lung fluid appears radiographically as a diffuse haziness in both lungs or an increase in perihilar markings. In ca. 50% of cases the small right interlobar fissure is visible on the first day, but as fluid absorption progresses it becomes less pronounced or may disappear completely. In a normal inspiratory view the diaphragm leaflets are projected over the posterior portions of the eighth and ninth ribs and the anterior portion of the sixth rib.
A. Smets and C. Schaefer-Prokop
Normal Thoracic Findings in Newborns
A. Smets and C. Schaefer-prokop
Findings | Possible misinterpretations |
Skin folds | Pneumothorax |
Air in the suprasternal notch | Esophageal atresia |
Funnel chest | Pneumomediastinum or cardiomegaly |
Oblique projection that omits the pectoral muscle | Unilateral hyperlucent lung |
Apparent opacity in the right paracardiac area | Infiltration |
Nipple-areola | Nodular mass |
Sternal ossification shifted from the midline due to an oblique projection | Nodular mass |
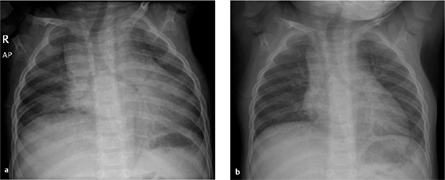
Expiratory radiograph (e. g., due to crying, evidenced by elevation of the diaphragm and tracheal bowing to the right) | Diffuse infiltration based on increased lung density, heart failure (Fig. 6.1) |
Fig. 6.1 a, b Misinterpretation due to poor inspiration.
A poor inspiratory view (a) cannot exclude bilateral pulmonary opacities and cardiomegaly. The findings disappear at full inspiration (b).
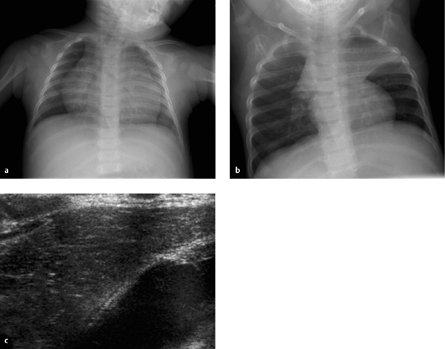
Fig. 6.2 a–c The neonatal thymus.
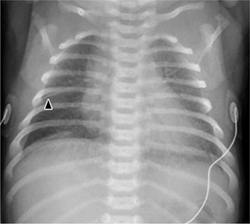
The thymus has the same density as the heart. It should not be misinterpreted as cardiomegaly (a), and its typical “sail” configuration should not be mistaken for atelectasis (b). On ultrasound scan, the thymus shows moderate homogeneous echogenicity with small white spots.
Thymus. The thymus appears as a hoodlike structure overlapping the cardiac silhouette and should not be mistaken for cardiomegaly (Fig. 6.2). Typically the thymus has the same radiographic density as the heart. The pulmonary vessels are visible within the thymic shadow much as they are in the retrocardiac space. On the ultrasound scan the thymus presents a characteristic homogeneous echo pattern with multiple interspersed “white dots” that resemble snowflakes.
The thymus is relatively large through 4 years of age (with considerable anatomic variation) and undergoes a steady involution after 9 years. Even if the thymus appears large on radiographs and covers the cardiac border and hila, normally it will never compress or displace mediastinal structures. This serves to distinguish a normal thymus from pathologic thymic dysplasia or a mediastinal tumor, which may become symptomatic if they compress or displace vessels or respiratory passages.
Cardiac size. Measurements for estimating cardiac size, like those performed in adults, are much less useful in children due to variables introduced by the thymus and variations in inspiratory position (a cardiothoracic ratio < 0.65 can serve as a guideline).
During Mechanical Ventilation
The tip of the endotracheal tube should be above the carina at the level of the T 2 vertebral body. The chin and head position should be noted during imaging: When the head is flexed, the tip of the tube is about 1 cm above the carina. Extending the head or tilting it to one side raises the tip to approximately the level of T1 or the head of the clavicle.
In infants with normal lung expansion during mechanical ventilation (e. g., high-frequency oscillatory ventilation), the right hemidiaphragm is projected between the posterior portions of the eighth and ninth ribs.
Catheter Position: Normal Findings and Malposition
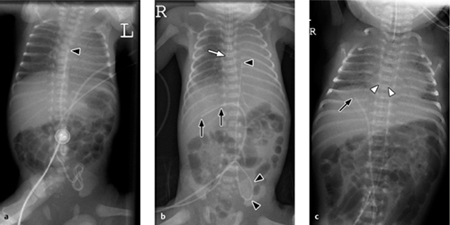
The anatomy of the fetal circulation is shown schematically in Fig. 6.3. The umbilical artery catheter (UAC) initially descends into the lesser pelvis. It enters the systemic circulation through the internal iliac artery or common iliac artery and runs toward the aorta. Its tip may be positioned above the aortic bifurcation, distal to the origin of the renal arteries (“low position” at the L3/L4 level), or at the level of the midthoracic aorta above the origins of the visceral arteries (“high position” at the T7/T 8 level). The catheter tip should not be located between the origins of the visceral or renal arteries (“no position” between T 10 and L 3 levels) (Fig. 6.4).
Fig. 6.3 Anatomy of the fetal circulation.
The umbilical vein arises from the placenta and opens into the inferior vena cava via the ductus venosus. The umbilical artery arises from the internal iliac artery and runs back along the bladder to the placenta. Because of this arrangement, an umbilical arterial catheter (UAC) first descends into the lesser pelvis whereas an umbilical venous catheter (UVC) runs cephalad to the liver.
1 Placenta
2 Bladder
3 Right lobe of liver
4 Left lobe of liver
5 Right lung
6 Left lung
7 Umbilical artery
8 Iliac artery
9 Aorta
10 Umbilical vein
11 Iliac vein
12 Ductus venosus
13 Inferior vena cava
14 Right ventricle
15 Left ventricle
16 Right atrium
17 Left atrium
18 Superior vena cava
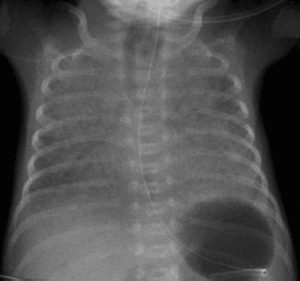
The umbilical vein catheter (UVC) runs directly upward through the ductus venosus and left portal venous system into the inferior vena cava. The tip should be just below the diaphragm, occupying a level that is cranial to the level of the liver veins and caudal to the right atrium (Fig. 6.4). The catheter tip should not be intrahepatic, as it might cause liver injury (Fig. 6.5).
Irrespective of whether the central venous catheter has been introduced through the inferior or superior vena cava, the tip should be just outside the right atrium. The same applies to the very thin peripheral venous catheters (silastic catheters), which are difficult to locate radiographically because of their small diameter. Their tip should also be outside the right atrium.
The tip of the gastric tube should be below the diaphragm!
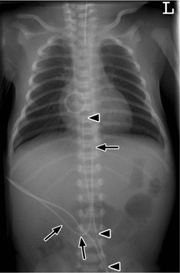
Fig. 6.4 Normal catheter position in a newborn.
The umbilical artery catheter (UAC, arrowheads) first dips into the lesser pelvis. Its tip is placed at the level of the T8 vertebra (high position). The umbilical vein catheter (UVC, arrows) runs directly cephalad with its tip just below the diaphragm.
Fig. 6.5 a–c Malpositioned catheters.
a The UAC is positioned too high (T5 level).
b The UVC is in the portal vein. The endotracheal tube is in the right main bronchus, causing atelectasis of the left lung. The gastric tube and UAC are correctly positioned.
c The UVC is in the portal vein, and the UAC is correctly positioned. The gastric tube is looped in the stomach, and the endotracheal tube is correctly positioned.
Transient Tachypnea of the Newborn (Wet Lung)
This condition (TTN or wet lung) is characterized by its transient nature with a typical correlation between the history, clinical manifestations, and radiographic findings. It most commonly occurs in preterm infants or children delivered swiftly or by cesarean section, which results in little or no opportunity for natural mechanical expulsion of the fetal fluid from the lung during delivery. Venous and lymphatic reabsorption of the fetal lung fluid is delayed, impairing the distensibility of the lungs. Transient left-sided heart failure may also play a role.
The diagnosis is suggested by the transient nature of the condition, continuous clinical and radiographic improvement within hours to a maximum of 2–3 days, and the relatively good clinical condition of the affected newborn.
Clinical Aspects
Children with TTN develop a (usually) mild respiratory dysfunction with tachypnea (90–140 breaths/min) during the first 6 hours of postnatal life (the normal range is up to 90 breaths/min). Oxygen may be administered by nasal cannula for a brief time, but generally the children are not intubated (in contrast to infantile respiratory distress syndrome [IRDS]).
Imaging
Radiographs taken during the first 2–6 hours of postnatal life usually show a symmetrical, hazy pattern of interstitial fluid accumulation in the lungs, small pleural effusions, and thickened septa outlined by fluid (Fig. 6.6). The heart is of normal size or may show transient slight enlargement. The lung volume is normal or slightly increased.
Fig. 6.6 Child with wet lung 6 hours after cesarean delivery.
Chest radiograph shows hazy lung opacity and a fluid-outlined septum on the right side. The lung volume is normal (the child is not intubated).
Reabsorption of the opacities begins peripherally and spreads centrally. As a result, radiographs taken ca. 10–12 hours after birth show increased symmetrical, linear markings in the perihilar region. All radiographic signs normally resolve within a maximum of 72 hours without additional therapeutic efforts.
Differential Diagnosis
Rapid improvement and the relatively mild degree of respiratory dysfunction are the main criteria that distinguish wet lung from neonatal pneumonia and hyaline membrane disease (see Table 6.2).
Infantile Respiratory Distress Syndrome
Synonyms for IRDS are surfactant deficiency disease (SDD) and hyaline membrane disease (HMD).
There is some confusion of terminology in that several terms are applied to the same condition. One states the cause (SDD), one the result (HMD), and another the resulting functional deficit (IRDS).
Respiratory distress syndrome is a common disease of preterm infants (< 37 weeks gestation, < 2500 g) caused by immaturity of the lungs and is the leading cause of death in this group. Surfactant deficiency prevents adequate expansion of the acini, leading to alveolar collapse and diffuse microatelectasis with impairment of gas exchange.
Imaging
Staging. Radiographs show general hypovolemia (!) of both lungs and a diffusely granular lung structure with an air bronchogram extending far into the periphery. Atelectasis is classified into four stages of severity (Table 6.3), although the findings are usually masked by immediate intubation and surfactant therapy (see below). Morphologic changes are often more pronounced in the lower lobes than in the upper lobes (Fig. 6.7).
Stage | Characteristics |
Stage I | Reticulogranular opacities, hypovolemia |
Stage II | Like stage I, plus an air bronchogram (the large bronchi do not collapse) |
Stage III | Like stage II, plus blurring of the cardiac border (increasing alveolar opacities) |
Stage IV | Homogeneous opacity of both lungs (white lung) |
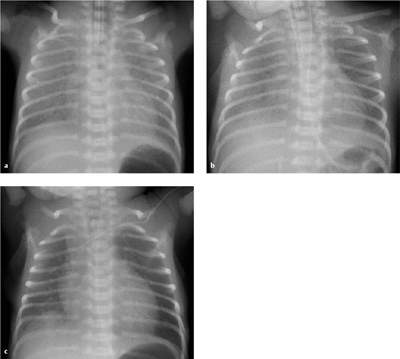
Fig. 6.7 a–d Different children with IRDS.
All of these infants are intubated and show pulmonary hypovolemia.
a Stage I–II: fine granular opacities with an air bronchogram (1 day after birth).
b Stage II: like stage I, but with a more conspicuous air bronchogram (1 day after birth).
c Stage III: like stage II, but with increasing unsharpness of the cardiac border (1 day after birth, same child as in a).
d Stage IV: white lung and air bronchogram.
Fig. 6.8 a–c Preterm infant with IRDS on ventilation.
a After birth.
b Ten hours after surfactant therapy.
c Two days after surfactant therapy. Note that improvement is more pronounced in the left lung. This asymmetry results from uneven intrapulmonary distribution of the surfactant.
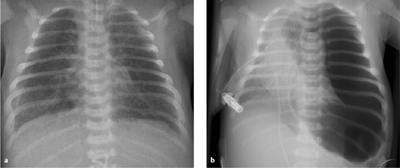
Fig. 6.9 a, b Meconium aspiration.
a Radiograph after meconium aspiration shows diffuse pulmonary hyperinflation (flattened diaphragm leaflets) with patchy opacities (1 day after birth).
b Massive tension pneumothorax after meconium aspiration in a different child (1 day after birth).
Course. Affected infants who are not intubated immediately after birth develop full-blown IRDS within ca. 12–24 hours. Today, however, it is rare to encounter fully developed IRDS or witness its staged progression because affected infants are intubated immediately after birth and surfactant is administered through the endotracheal tube, often without prior imaging. Surfactant therapy in responders leads to increased lung volumes and clearing of granular opacities (Fig. 6.8), at which point the findings may resemble pulmonary interstitial emphysema. With an uneven intrapulmonary distribution of surfactant (e. g., right > left), radiographic findings may be very asymmetrical and mimic the appearance of neonatal pneumonia or meconium aspiration (see Fig. 6.9). These cases require close cooperation with the pediatrician to ensure that the images are interpreted correctly.
Differential Diagnosis
Pleural effusion is not a feature of IRDS and would be more consistent with neonatal pneumonia. Resolution of radiographic findings within a few hours would also be inconsistent with IRDS. Reappearance of pulmonary haziness after an interval of improvement is a sign of pulmonary edema (see also Table 6.2).
Meconium Aspiration Syndrome
The intrauterine or intrapartum aspiration of meconium-stained amniotic fluid occurs mainly in children who are delivered several days postterm, or in patients where intrauterine or perinatal stress has caused vagal stimulation leading to increased intestinal peristalsis and premature passage of meconium into the amniotic sac.
Meconium aspiration syndrome (MAS) is a very serious condition. Many infants with MAS have pulmonary dysfunction that is severe enough to require extracorpo-real membrane oxygenation (ECMO).
Imaging
The radiographic changes caused by the aspiration of meconium or amniotic fluid with a high cellular content are highly variable. The following signs may be found, depending on the site of the bronchial obstruction and quantity of aspirated fluid:
 An asymmetric hyperinflation of one or both lungs or lung areas with associated depression of the hemidiaphragm (Fig. 6.9a)
An asymmetric hyperinflation of one or both lungs or lung areas with associated depression of the hemidiaphragm (Fig. 6.9a)
 Perihilar linear opacities with a radial distribution
Perihilar linear opacities with a radial distribution
 Coarse, sometimes nodular opacities surrounded by cystlike lung areas correspond to focal zones of atelectasis surrounded by areas of compensatory hyperinflation
Coarse, sometimes nodular opacities surrounded by cystlike lung areas correspond to focal zones of atelectasis surrounded by areas of compensatory hyperinflation
Up to 40% of patients have a (small) effusion and an associated pneumothorax, the latter as a complication from the combination of airway obstruction and hyperinflation, plus the need for relatively aggressive suctioning (Fig. 6.9b). Hypoxemia and hypercapnia lead to pulmonary vasoconstriction with secondary pulmonary hypertension.
Rarely, children show no pulmonary opacities at all after meconium aspiration but develop solely a pneumothorax: they usually have such poor respiratory function that mechanical ventilation is required.
Course. A chemical pneumonitis may develop within 48 hours, especially when large amounts of meconium have been aspirated. Secondary infections are common; the main causative organisms are gram-negative bacteria. Secondary surfactant deficiency may lead to a secondary respiratory distress syndrome that is difficult to distinguish radiographically from neonatal pneumonia.
Neonatal Pneumonia
Preterm infants are at particularly high risk for developing neonatal pneumonia. The infection may be acquired in utero (e. g., transplacental listeriosis or CMV infection) or during delivery, due to aspiration of infected amniotic fluid. The causative agents are more often bacterial than viral. Even mature newborns may develop neonatal pneumonia.
Imaging
Because the interstitium predominates over aerated parenchyma in newborns, the initial response of the neonatal lung to various pathogens is an increased density of interstitial structures (Fig. 6.10). The most frequent type of infection is streptococcal pneumonia, but other causative agents such as staphylococci, fungi, and viruses also occur.
Fig. 6.10 Neonatal pneumonia in a 5-day-old infant.
Note the diffuse alveolar opacity. (Differentiating features from IRDS: child is not intubated, IRDS occurs in the immediate post-natal period.)
Course. Subsequent radiographs show patchy, usually bilateral and sometimes confluent foci in addition to diffuse alveolar opacities. The opacities may show an asymmetrical arrangement..
Usually significant hyperinflation of the lungs is present. Mild cardiomegaly is another common finding.
Differential Diagnosis
The findings are difficult to distinguish from IRDS. The most important criterion is the presence of pleural effusion that is more frequently seen in infants with neonatal pneumonia. Note that IRDS and neonatal pneumonia may also coexist.
Complications during or after Mechanical Ventilation
Pulmonary Interstitial Emphysema
Pulmonary interstitial emphysema (PIE) is a result of mechanical ventilation using a high transpulmonary pressure (barotrauma). The high pressure causes overexpansion of the terminal bronchioles and alveolar ducts.
The diagnosis of PIE has special implications for ventilation therapy (high-frequency ventilation) (Table 6.4) and is considered a precursor of other, more serious complications such as pneumothorax and pneumomediastinum. Frequent radiographic follow-ups are needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 The normal thymus is relatively large through age 4 years but never compresses or displaces mediastinal structures.
The normal thymus is relatively large through age 4 years but never compresses or displaces mediastinal structures. The tip of the endotracheal tube is ca. 1 cm above the carina when the head is flexed, rising to the level of T1 or the head of the clavicle when the head is extended or tilted to one side.
The tip of the endotracheal tube is ca. 1 cm above the carina when the head is flexed, rising to the level of T1 or the head of the clavicle when the head is extended or tilted to one side.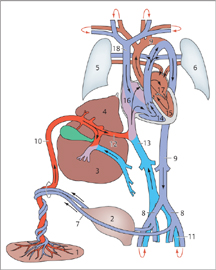
 TTN is a transient condition marked by rapid clinical and radio-graphic improvement and relatively mild respiratory dysfunction.
TTN is a transient condition marked by rapid clinical and radio-graphic improvement and relatively mild respiratory dysfunction.
 IRDS, in which surfactant deficiency prevents adequate expansion of the acini, is the leading cause of death in preterm infants (< 37 weeks, < 2500 g).
IRDS, in which surfactant deficiency prevents adequate expansion of the acini, is the leading cause of death in preterm infants (< 37 weeks, < 2500 g).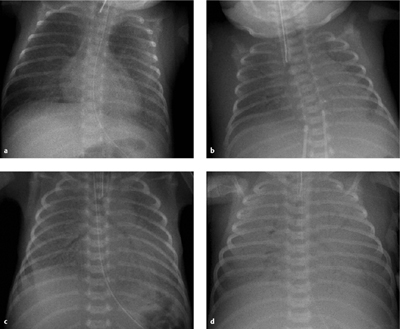
 The aspiration of meconium-stained amniotic fluid leads to pulmonary dysfunction, which may be severe enough to require ECMO.
The aspiration of meconium-stained amniotic fluid leads to pulmonary dysfunction, which may be severe enough to require ECMO. The neonatal lung responds initially to bacterial infection with an increased density of interstitial structures.
The neonatal lung responds initially to bacterial infection with an increased density of interstitial structures.