First author
Publication year
Hybrid system
N
Reference standard for definition of significant CAD
Sens
Spec
PPV
NPV
Namdar
2005
13N-NH3 PET/4-slice CCTA
25
Flow-limiting coronary stenoses requiring revascularization (ICA + PET)
90
98
82
99
Rispler
2007
SPECT/16-slice CCTA
56
Flow-limiting coronary stenoses (>50 % stenosis on ICA + SPECT pos.)
96
95
77
99
Groves
2009
82Rb PET/64-slice CCTA
33
>50 % stenosis on ICA
88
100
97
99
Sato
2010
SPECT/64-slice CCTAa
130
>50 % stenosis on ICA
94
92
85
97
Kajander
2010
15O-H2O PET/64-slice CCTA
107
Flow-limiting coronary stenosis (>50 % stenosis of ICA + FFR)
93
99
96
99
Schaap
2013
SPECT/64-slice CCTA
98
Flow-limiting coronary stenosis (>50 % stenosis of ICA + FFR)
96
95
96
95
Danad
2013
15O-H2O PET/64-slice CCTAb
120
Flow-limiting coronary stenosis (>50 % stenosis of ICA + FFR)
76
92
86
84
Thomassen
2013
15O-H2O PET/64-slice CCTA
44
>50 % stenosis on ICA (QCA)
91
100
100
92
Overall, the diagnostic accuracy of fusion imaging is very high with a sensitivity, specificity, positive predictive value and negative predictive value of 76–96 %, 92–100 %, 77–100 %, and 84–99 %, respectively. The incremental value of fusion cardiac imaging lies in the accurate spatial co-localization of myocardial perfusion defects assessed by SPECT or PET and subtending coronary arteries (Fig. 10.1). However, there are some limitations which apply to these early clinical studies such as the difference in hybrid systems, the variable reference standard (either invasive coronary angiography alone or in combination with SPECT or FFR), the single-center design, as well as the small sample size.
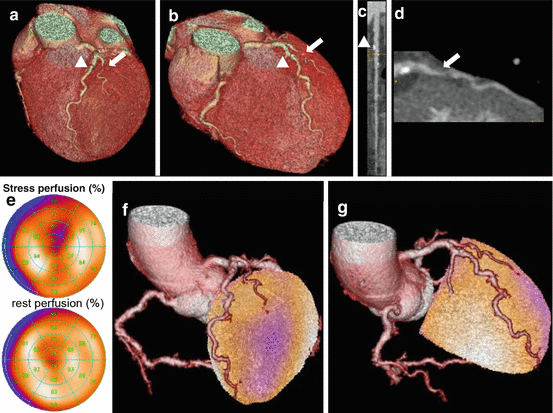

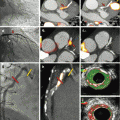
Fig. 10.1
Panel (a, b) show different views of a cardiac 3D CCTA reconstruction documenting an intermediate stenosis in the proximal LAD (arrowhead) as well as an intermediate stenosis without calcification (soft plaque) in the first diagonal branch (DA, arrow). Curved multiplanar reformation of the LAD and the first DA are seen in Panels c and d, respectively. SPECT MPI (Panel e) reveals a reversible anteroapical perfusion defect, which can be accurately attributed to the corresponding territory of the first DA by fused SPECT/CCTA images (Panel f, g)
In the first clinical study documenting the feasibility and clinical robustness of noninvasive fusion imaging after fusion of 13N-NH3 PET with four-slice CCTA in 25 patients with CAD, the hybrid PET/CCTA images allowed identifying flow-limiting coronary lesions requiring a revascularization procedure (as defined by ICA and PET) with a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 90 %, 98 %, 82 %, and 99 %, respectively (Table 10.1) [13]. Similar results were documented in a study by Rispler and coworkers, who were able to show an increase in specificity (from 63 to 95 %) and PPV (from 31 to 77 %) with SPECT/CCTA compared to CCTA alone [14]. Another study by Sato and coworkers demonstrated a particular improvement in specificity and PPV by adding SPECT information in non-evaluable arteries on CCTA (from 80 to 92 % and from 69 to 85 %, respectively) [15]. Kajender and coworkers demonstrated in a study of 107 patients who underwent 15O-H2O PET/64-slice CCTA that hybrid images increased the PPV significantly from 76 to 96 % compared to CCTA alone [16]. Moreover, in that study, the hemodynamic significance of ICA stenoses was confirmed by fractional flow reserve (FFR) in 18 of 40 patients providing a more comprehensive reference standard. Similarly, Schaap and coworkers showed an improvement of specificity from 62 and 79 % when using CCTA alone and SPECT alone, respectively, to a specificity of 95 % in a combined approach [17]. In a study using 15O-H2O PET/64-slice CCTA on 120 patients with an intermediate likelihood for CAD, the diagnostic accuracy of quantitative 15O-H2O PET/64-slice CCTA was superior to either 15O-H2O PET or CCTA alone [18]. Finally, in a study by Thomassen and coworkers, the quantitative hybrid PET/CCTA imaging yielded an excellent sensitivity, specificity, positive predictive value, and negative predictive value of 91, 100, 100, and 92 % pointing out the benefit especially in increasing the positive predictive value, which was 71 % for CCTA alone and 87 % for SPECT alone [19].
These studies have documented the incremental diagnostic value of fusion imaging compared to one imaging modality alone. Furthermore, so far, there have been three studies which have specifically evaluated the additional value of fusion cardiac imaging over the side-by-side analysis of CCTA and myocardial perfusion images. In the first study, the number of lesions with equivocal hemodynamic relevance could be significantly reduced using SPECT/CCTA fusion compared with the side-by-side analysis in 38 patients with perfusion defects on SPECT [20]. In another study conducted by Santana and coworkers, a higher diagnostic performance for fused SPECT/CCTA imaging compared to the side-by-side analysis of SPECT and CCTA (p = 0.007) for the diagnosis of obstructive CAD on ICA could be demonstrated [21]. This was mainly true in patients with multivessel disease. Finally, Slomka and coworkers demonstrated an improved diagnostic value of cardiac fusion imaging mainly in the territories of the circumflex artery and the right coronary artery in a study implementing motion-frozen SPECT data and CCTA-guided SPECT contour and territory adjustments [22].
In summary, cardiac fusion imaging combining the anatomic information from CCTA with the functional information from myocardial perfusion imaging offers an incremental diagnostic value in the identification of hemodynamically significant coronary artery stenoses and thereby in guiding clinicians on the appropriate treatment (medical versus revascularization). The added value has not only been proven when comparing to one imaging modality alone, but also when comparing to the side-by-side analysis.
10.3.2 Clinical Indication for Fusion Imaging
As the combined hybrid approach is associated with increased radiation burden as well as higher costs, it is of great importance to select patients carefully. It is still an open question which patients do truly benefit from such an integrated approach. For the vast majority of patients referred for noninvasive testing, a single imaging method (either CCTA or myocardial perfusion imaging depending on the clinical situation) is sufficient. In the following clinical scenarios, the hybrid approach might be helpful.
10.3.2.1 Intermediate Pretest Likelihood
It is essential to assess the pretest likelihood in every patients presenting with symptoms of CAD. The main determinants of likelihood are age, gender, characteristics of chest pain, and ECG changes during exercise test. In patients with an intermediate pretest likelihood, the probability of an inconclusive or intermediate result in either of the imaging method is higher. Thus, in such situations, a combined approach is helpful to determine the relevance of a given lesion. For example, in a patient with an intermediate stenosis in CCTA, the subsequent assessment of the functional significance by myocardial perfusion imaging is needed to decide whether the patient can be treated medically or should undergo invasive revascularization. There are several centers with hybrid imaging facilities performing such an integrated approach sequentially.
10.3.2.2 Significant Side-Branch Disease
Although side branches (diagonal and posterolateral branches) of main coronary arteries are often seen as clinically irrelevant, patients may still suffer from symptoms such as angina as these side branches may induce ischemia. In such situations, fusion images provide an accurate method to assess the hemodynamic significance of such lesions, especially as the coregistration allows to exactly determine the vessel causing the ischemia.
10.3.2.3 Multivessel Disease
It is well known that patients with multivessel disease (MVD) are at increased risk for future adverse cardiac events. Although angiographically suspected, a large number of patients with MVD do not have any perfusion abnormality. Studies have shown that fusion imaging has an incremental diagnostic value compared to perfusion imaging alone, particularly in patients with MVD. Moreover, fusion imaging allows to accurately detect the culprit lesion, so that fusion imaging can been seen as a “gatekeeper” for ICA and revascularization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree