31.6 Imaging
Imaging has an essential role in the diagnosis, staging, and follow-up of thymic malignancies. Imaging is used to initially identify the tumor and then stage it properly, with emphasis on local invasion and distant spread to help identify those patients needing neoadjuvant therapy (stage III and IVa) prior to surgery. The role of imaging following initial treatment is to identify recurrence while it is still resectable, since complete resection of recurrent tumor obtains outcomes similar to those for patients without recurrence [24, 25].
31.6.1 Normal Thymus and Thymic Hyperplasia
The appearance of the normal thymus, which at times may be enlarged, should be recognized and not confused with thymoma on imaging studies. In young children, the normal thymus and thymic hyperplasia may at times mimic a mediastinal mass. The normal adult thymus is not detected by chest radiography but exhibits a triangular or arrowhead-shaped appearance on CT and MRI and is best evaluated at the level of the aortic arch. CT imaging of thymic hyperplasia typically shows a diffusely enlarged thymus with smooth borders. Thymic hyperplasia manifesting as a mass on CT or MR is differentiated from a primary mediastinal neoplasm on the basis of the diffuse, symmetric enlargement with preservation of the normal shape of the thymus seen in hyperplasia (Fig. 31.1). Similar findings distinguish rebound hyperplasia, which occurs 3–8 months after cessation of chemotherapy in approximately 25 % of patients [26] treated for malignancy. When CT and conventional MR imaging sequences cannot differentiate a normal thymus or thymic hyperplasia from a thymoma, chemical shift MR imaging sequences (in-phase and out-of-phase gradient echo sequences) can be helpful. Chemical shift MR imaging sequences identify the normal fatty infiltration of the normal or hyperplastic thymic tissue. This infiltration manifests as a homogeneous signal decrease on out-of-phase images relative to in-phase images, in contrast to tumors, which do not show a signal decrease [27].
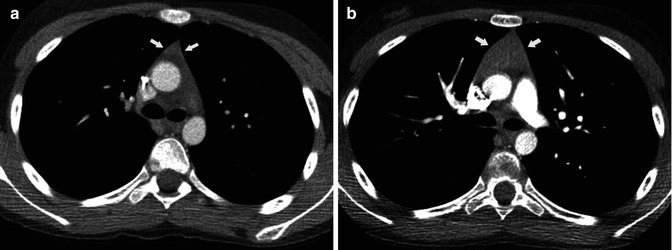

Fig. 31.1
Fourteen-year-old woman with bilateral ovarian dysgerminoma. (a) Contrast-enhanced chest CT during chemotherapy treatment shows the triangular morphology of the normal thymus (arrow). (b) Contrast-enhanced chest CT 3 months after completion of chemotherapy shows diffuse thymic enlargement with preservation of its triangular shape consistent with thymic hyperplasia (arrow)
31.6.2 Radiography
Radiographically, a thymoma typically manifests as a unilateral, well-marginated anterior mediastinal mass with smooth or lobulated contours that is located anywhere from the thoracic inlet to the cardiophrenic angle (Fig. 31.2). When the mass is relatively small, evaluation of the anterior junction line or identification of the mass in the retrosternal region on the lateral chest radiograph is helpful. Chest radiographic signs of locally advanced disease include irregular tumor borders with adjacent lung and phrenic nerve involvement as well as associated elevation of the hemidiaphragm. Evidence of pleural implants (Fig. 31.3) is characteristic of metastatic involvement (stage IVa). Thymic neuroendocrine tumor and thymic carcinoma are more aggressive, and a chest radiograph usually shows a large poorly defined anterior mediastinal mass. Pleural and lymph node mediastinal involvement is not uncommon.
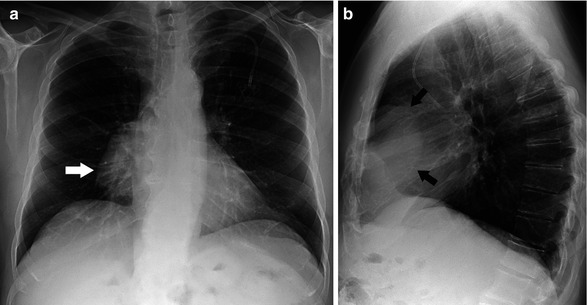
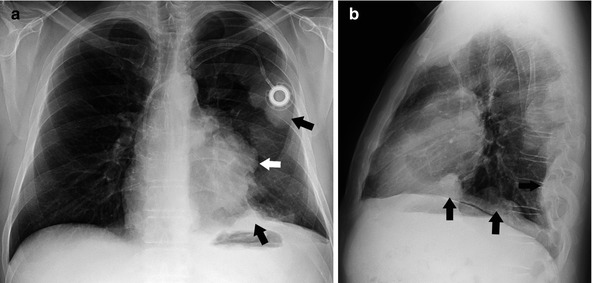

Fig. 31.2
Asymptomatic 65-year-old man with invasive thymoma. Posteroanterior (a) and lateral (b) chest radiographs demonstrate a large unilateral right anterior mediastinal mass with lobular contours (arrow)

Fig. 31.3
Fifty-one-year-old man presenting with chest pain. Posteroanterior (a) and lateral (b) chest radiographs show a left anterior mediastinal mass (white arrow) with multiple left pleural nodular thickening (black arrow) consistent with surgically proven pleural metastatic involvement
31.6.3 Computed Tomography
CT is the primary imaging modality used in the diagnosis, staging, and treatment management of thymic malignancies. CT not only is superior to chest radiography in evaluating tumor extent but also is used to help distinguish thymoma from other diseases that may manifest in the anterior mediastinum. The presence of extensive mediastinal lymphadenopathy, pleural effusions, and pulmonary metastases is not common in thymoma. The presence of any of these should raise the possibility of other malignancies, including lymphoma, thymic carcinoma, and lung cancer or even of metastatic involvement due to abdominal neoplasms. Thymomas typically manifest as a 1–10 cm (mean, 5 cm) mass with smooth or lobulated contours that characteristically arises from one lobe of the thymus (Fig. 31.4). Although thymomas typically present as unilateral masses, bilateral mediastinal involvement can occur [28]. Homogenous uptake is usually seen in about two thirds of thymomas, although necrosis, cystic change, or hemorrhage may produce some heterogeneity. When attenuation is low, the presence of soft tissue nodularities should alert the radiologist that one is dealing with a cystic neoplasm rather than a congenital cyst [28]. This nodularity is more readily identified with the use of intravenous contrast. Multiple patterns of calcification may be seen and can be thin, linear, and located in the capsule. Most thymomas are completely encapsulated neoplasms, but 30–60 % show various degrees of invasion of the tumor capsule and the adjacent structures [10, 29, 30].
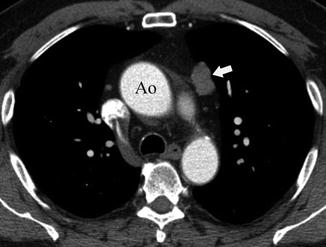

Fig. 31.4
Sixty-two-year-old man with an incidental finding in a CT screening study. Contrast-enhanced chest CT at the level of the ascending aorta (Ao) shows a 2.5 cm left anterior mediastinal mass (arrow). A thymoma with microscopic tumor capsular invasion was diagnosed at pathology (stage IIA)
Vascular invasion, pleural involvement, and pericardial dissemination are typical of advanced stage disease. Direct signs of vascular involvement may manifest as irregular luminal contours, vascular encasement (Fig. 31.5) or obliteration, or evidence of endoluminal soft tissue density, which may extend into the cardiac chambers [28]. Pleural involvement (“drop metastases”) is characterized on CT as an isolated pleural nodularity that can be smooth, irregular, or diffuse but is almost always ipsilateral to the anterior mediastinal tumor (Fig. 31.6). Pleural effusion is uncommon, even in the presence of extensive pleural metastatic disease [31]. Over the years, there was agreement that CT had a limited capacity to detect tumor invasiveness even though few published studies compared CT appearance and Masaoka-Koga staging. In a retrospective study assessing 50 thymoma patients, Tomiyama et al. [32] sought to distinguish stage I thymoma from more advanced disease (stages II–IV). In that study, partial or complete obliteration of fat planes around the tumor was not helpful in distinguishing stage I from higher stages. Lobulated or irregular contours, cystic or necrotic portions within the tumor, and multifocal calcification were, however, more suggestive of invasive (stage II and higher) thymoma [32]. Another study comparing the CT appearance of thymoma in 99 patients tried to differentiate stages I and II thymomas from stages III and IV. That study found that CT was useful in distinguishing stage I and II thymomas from more advanced disease (stages III and IV) [33]. The CT characteristics that showed a significant association with invasive (stage III or IV) disease were primary tumor size ≥7 cm, infiltration of the fat surrounding the tumor, and lobulated tumor contours [33]. Although according to one study, the WHO classification was found to be insufficiently reproducible and to lack clinical predictive value [16], several CT studies have correlated CT appearance with the WHO histologic classification. Two studies that evaluated patients with thymoma [34, 35] found that lobulated and irregular tumor contours were associated with more aggressive disease although that finding was not confirmed by a third study [36]. Thymic carcinomas demonstrate an anterior mediastinal mass with irregular or lobulated contours. Attenuation is heterogeneous frequently with areas of hemorrhage, necrosis, or cystic changes (Fig. 31.7). Calcifications may appear in 10–40 % of cases.
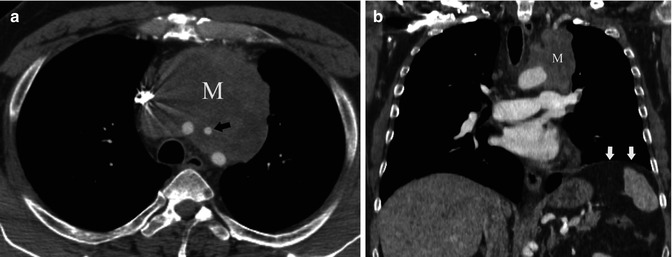
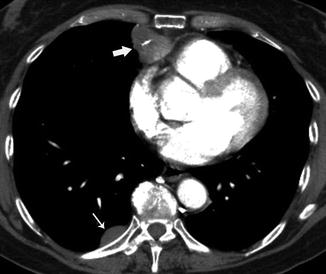
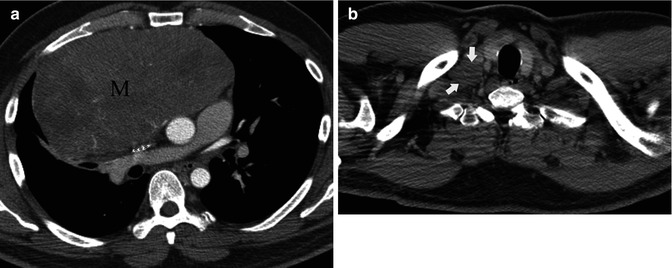

Fig. 31.5
Fifty-nine-year-old man with cough. (a) Contrast-enhanced chest CT demonstrates a right anterior mediastinal mass (M) surrounding great vessels, most evident at the level of the left carotid artery (arrow). (b) Chest CT coronal reformation shows a lobulated left mediastinal mass (M) and elevation of the ipsilateral hemidiaphragm (arrows). Invasive thymoma with vascular and phrenic nerve involvement was diagnosed at surgery (stage III)

Fig. 31.6
Fifty-seven-year-old woman with myasthenia gravis and chest pain. Contrast-enhanced chest CT shows an anterior mediastinal mass (thick arrow) with nodular pleural implants, called “drop metastasis” in the right side (thin arrow). Thymectomy and resection of pleural implants were performed surgically (stage IVA)

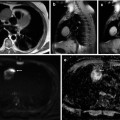
Fig. 31.7
Asymptomatic 43-year-old man. (a) Contrast-enhanced chest CT demonstrates a large right anterior mediastinal mass (M) compressing the superior vena cava . (b) An additional axial CT slice at the level of the thoracic inlet shows a right supraclavicular adenopathy (arrows) consistent with nodal metastatic disease. Thymic carcinoma was diagnosed at surgery (stage IVB)
31.6.4 Magnetic Resonance Imaging
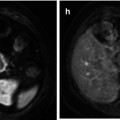
With advances in multi-detector CT technology allowing for 3D reformatting, the use of MRI in the evaluation of thymic neoplasms has decreased. However, MR may be used to assess local tumor invasiveness when the use of iodine intravenous contrast is contraindicated (Fig. 31.8) as well as to decrease radiation dose and work as an alternative method in evaluation of thymic malignancies. On MR images, thymomas manifest with low to intermediate signal intensity (similar to skeletal muscle) on T1-weighted images and high signal intensity on T2-weighted images (Fig. 31.9), which may exhibit signal intensity levels approaching that of fat [37, 38]. To differentiate surrounding fat from thymoma, fat suppression techniques may be useful. Heterogeneous signal intensity is present in those tumors that have focal areas of necrosis, hemorrhage, and cystic change. Cystic changes and intratumoral necrosis manifest as areas of low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Additionally, fibrous septa within the tumor can be seen as low signal intensity regions. Hemorrhage is identified as high signal intensity on T1- and T2-weighted images due to hemosiderin deposition. Detection of mural nodules in intratumoral cystic degeneration is consistent with cystic neoplasm. Although CT is superior to MRI in demonstrating calcification within thymomas, MR imaging can occasionally reveal fibrous septa within the masses and permit better evaluation of the tumor capsule and the presence of hemorrhage [36]. Sakai et al. [39] studied 31 patients with thymoma, demonstrating that with dynamic MR imaging, mean peak time was delayed in advanced thymomas (stages III and IV) compared with earlier-stage tumors.
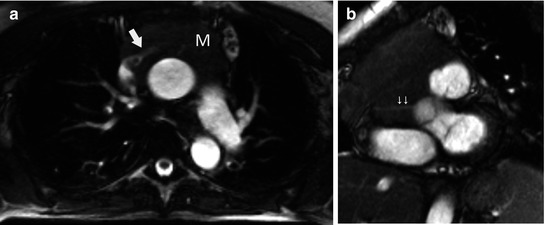
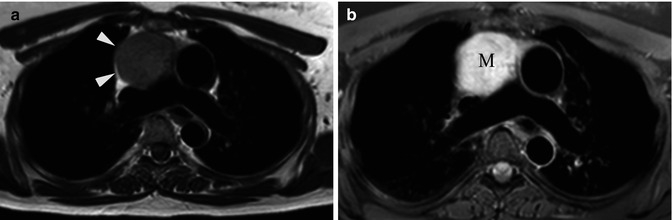

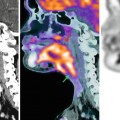
Fig. 31.8
Fifty-four-year-old woman with shortness of breath. Patient was allergic to iodine, and chest CT was not helpful to detect vascular involvement. (a) Axial FIESTA (fast imaging employing steady-state acquisition) MRI demonstrates upper anterior mediastinal mass (M) surrounding the left brachiocephalic vein (arrow). Note also tumor extension to the junction between the SVC and left brachiocephalic vein. (b) Oblique FIESTA MRI shows tumor extension to the right atrioventricular sulcus with encasement of the right coronary artery (arrow)

Fig. 31.9
Forty-seven-year-old woman with left shoulder pain. (a) Axial T1-weighted MR image shows rounded low signal intensity mass (arrowheads) in the anterior mediastinum. (b) Axial T2-weighted fat-suppressed MR image demonstrates a 5 cm anterior mediastinal mass (M) with high signal intensity. A stage I thymoma was diagnosed at resection
Thymic carcinoid tumors show typical bright signal intensity on T2-weighted images. Direct tumor invasion to adjacent mediastinal structures and vascular encasement are common findings in patients with thymic carcinomas.
31.6.5 Scintigraphy
Nuclear medicine techniques are not currently used for evaluation of thymic neoplasms. CT has replaced thallium 201 (201Tl) for differentiating among normal thymus, thymic hyperplasia, and thymic malignancies as nuclear medicine studies offer low special resolution and overlap criteria. Somatostatin receptors are overexpressed in neuroendocrine tumors, including other malignant thymic epithelial tumors [40]. Although somatostatin receptor scintigraphy using radiolabeled octreotide (OctreoScan) cannot differentiate between thymoma, thymic carcinoma, and thymic NET, it can be used for tumor identification, to distinguish from other anterior mediastinal malignancies such as lymphoma and germ cell tumors, and to exclude tumor involvement in another site that may have metastasized from the thymus [40] (Fig. 31.10). Additionally indium111 octreotide shows uptake most commonly in neuroendocrine tumors such as thymic carcinoids and is used to identify patients who may respond to treatment with octreotide [41].
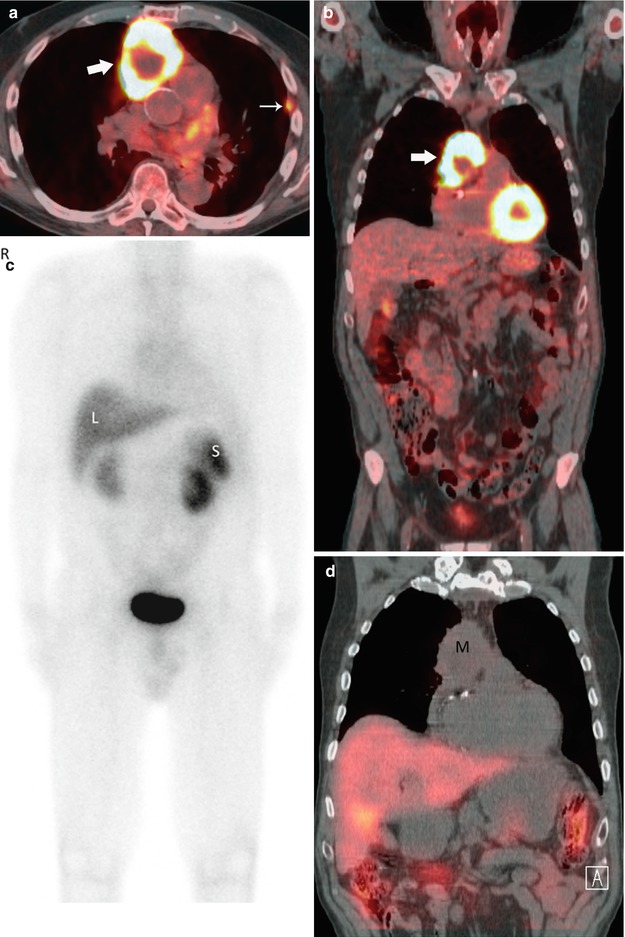

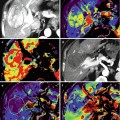
Fig. 31.10
Seventy-year-old man with diagnosis of thymic carcinoma. Axial (a) and coronal (b) fused PET-CT images show intense FDG uptake in an anterior mediastinal mass (thick arrow) with an SUVmax of 22.3. Note is also made of pleural implants (thin arrow) consistent with metastatic involvement. Nuclear medicine tumor localization using indium111 octreotide was obtained to consider use of octreotide as treatment. A coronal PET MIP (c) and CT fusion with coronal reformation (d) reveals no focal activity in the mediastinal tumor (M). Liver (L) and spleen (S)
31.6.6 Positron Emission Tomography with Fluorodeoxyglucose
Imaging with fluorodeoxyglucose (FDG) with integrated positron emission tomography (PET-CT) has been accurate for the clinical staging of intrathoracic malignancies, especially non-small cell lung cancer [42, 43]. This advantage of FDG-PET is less apparent in imaging thymic neoplasms, particularly in thymomas, and the role of PET-CT in patient management is a matter of controversy. One difficulty is that increased physiologic FDG uptake by the normal thymus or thymic hyperplasia is common, especially in children and young patients [44]. Studies assessing FDG-PET imaging include small sample sizes, the majority combining thymic cancers and thymoma. Despite the fact that no studies with a large number of patients have evaluated FDG-PET imaging of thymic malignancies, there is agreement in the literature that maximum Standard Uptake Value (SUVmax) in thymic carcinomas is significantly higher than in thymomas (Fig. 31.11), but these studies varied in their capacity to distinguish between low-risk and high-risk thymomas. Endo et al. [45] found significant differences in 18F-FDG accumulation for low-risk thymoma, high-risk thymoma, and thymic cancer. Sung et al. [46] and Kumar et al. [47] were able to differentiate thymomas from thymic carcinomas but were not able to differentiate low-risk tumors (WHO types A, AB, and B1) from high-risk tumors (type B2 and B3). However, the clinical dilemma imaging has to resolve is the distinction of stage III and IV thymoma from earlier-stage thymomas, which will affect therapy. Although it was hoped that functional imaging would be helpful to make this distinction, that result was unfortunately not confirmed, and FDG-PET should not be used to differentiate these groups. One study by Shibata et al. [48] of 40 patients with thymoma identified 17 tumors as stage I, 17 tumors as stage II, 4 tumors as stage III, and 2 tumors as stage IV, demonstrating that SUV cannot predict the invasiveness of thymomas assessed by tumor stage [48]. Another study included 37 thymoma patients who underwent FDG-PET-CT prior to surgery. The FDG uptake as assessed on the basis of SUVmax was variable and did not assist with distinguishing early stage (stages I and II) from advanced stage (stages III and IV) disease (p = 0.135) [49]. The ability of FDG-PET to distinguish between local recurrence and post-therapeutic changes remains an open question. El-Bawab et al. [50] suggested that PET-CT was helpful in distinguishing recurrent tumors in the mediastinum from fibrotic scar tissue in patients who underwent surgical excision of thymoma [50]. Eleven recurrent tumors were correctly identified by FDG-PET, whereas CT reported five false-negative and two false-positive cases. Additionally, the role of PET in predicting the amount of tumor response to chemotherapy is still unclear. Kaira et al. [51] studied 12 patients with unresectable thymic epithelial tumors, 10 patients with thymic carcinoma, and 2 patients with thymoma. Their results suggested that FDG uptake in primary tumors after therapy could predict the outcomes of patients with thymic carcinomas (p




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree