, David C. ShonkaJr.2, Prashant Raghavan3, Max Wintermark4 and Sugoto Mukherjee5
(1)
Division of Body Imaging, Department of Radiology and Medical Imaging, University of Virginia Health System, Charlottesville, VA, USA
(2)
Division of Head and Neck Surgical Oncology, Department of Otolaryngology – Head and Neck Surgery, University of Virginia Health System, Charlottesville, VA, USA
(3)
Division of Neuroradiology, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
(4)
Department of Radiology and Medical Imaging, University of Virginia Health System, Charlottesville, VA, USA
(5)
Division of Neuroradiology, Department of Radiology and Medical Imaging, University of Virginia Health System, Charlottesville, VA, USA
Abstract
The thyroid gland is an essential component of our endocrine system and regulates a wide range of critical metabolic functions. Both focal and diffuse thyroid disorders can impact function. The parathyroid glands regulate serum calcium levels. Parathyroid evaluation is almost always done in the setting of hyperparathyroidism and hypercalcemia. A combination of imaging techniques is used to evaluate thyroid and parathyroid pathology and function, including ultrasound, CT, MRI, and radionuclide imaging.
12.1 Introduction
The thyroid gland is an essential component of our endocrine system and regulates a wide range of critical metabolic functions. Both focal and diffuse thyroid disorders can impact function. The parathyroid glands regulate serum calcium levels. Parathyroid evaluation is almost always done in the setting of hyperparathyroidism and hypercalcemia. A combination of imaging techniques are used to evaluate thyroid and parathyroid pathology and function, including ultrasound, CT, MRI, and radionuclide imaging.
12.2 Anatomy
The thyroid gland consists of two lobes connected by midline isthmus. It lies in the visceral space, anteromedial to the carotid space, between the prevertebral muscles posteriorly and the infrahyoid strap muscles anteriorly (Fig. 12.1). An accessory pyramidal lobe can extend superiorly from the isthmus toward the hyoid bone. The tubercle of Zuckerkandl can often be reliably identified on CT and is located on the posterior aspect of each lobe; it serves as a surgical landmark for the recurrent laryngeal nerve, which passes posterior to it (Fig. 12.2).
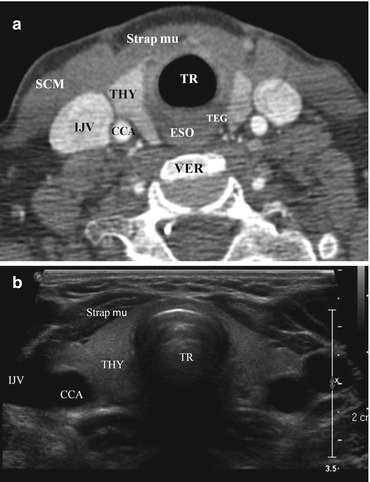
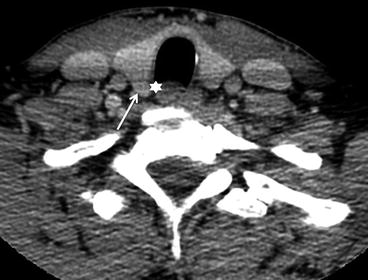

Fig. 12.1
Normal CT and US images of the thyroid gland. Contrast-enhanced axial CT (a) and axial grayscale US images (b) of the thyroid gland in the infrahyoid neck show the normal anatomical relationships of the relevant structures. These include the following: Strap mu strap muscles, SCM sternocleidomastoid muscle, IJV internal jugular vein, VER cervical vertebra, CCA common carotid artery, TEG tracheoesophageal groove, TR trachea, ESO esophagus, THY thyroid

Fig. 12.2
Zuckerkandl tubercle. The Zuckerkandl tubercle (also referred to as the posterior thyroid tubercle) is usually identified as a nodular posterior extension (arrow) of thyroid tissue beyond the tracheoesophageal groove (star). It serves as an important surgical landmark for the recurrent laryngeal nerve, which courses medial to it
The thyroid gland develops from the third and fourth pharyngeal pouches and descends during fetal development from the foramen cecum at the midline base of the tongue to the thyroid bed along the course of the thyroglossal duct (Fig. 12.3).
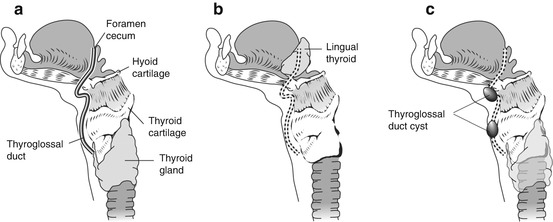

Fig. 12.3
Graphic showing the course of the thyroglossal duct cyst from the foramen cecum in the base of the tongue, passing anterior to the hyoid bone and laryngeal cartilages to its final destination in a pretracheal location (a). Abnormal descent of the thyroid gland or failure of obliteration of the thyroglossal duct can lead to ectopic thyroid tissue (b) and thyroglossal cysts (c) along the course of the thyroglossal duct
The thyroid gland is supplied by paired superior and inferior thyroid arteries, which arise from the external carotid and thyrocervical trunk, respectively. The thyroidea ima artery is present in 4–10 % of the population and can have a variable origin from the arch of the aorta or the innominate artery. The venous drainage is via the superior and middle thyroid veins into the internal jugular veins and the inferior thyroid veins into the brachiocephalic veins. The lymphatic drainage is to adjacent pretracheal, paratracheal, and prelaryngeal nodes in the visceral space (level VI). Regional drainage can occur to levels II–V in the lateral neck. The gland is closely associated with the superior and recurrent laryngeal nerves.
The thyroid gland uses iodine to produce thyroxine (T4) and triiodothyronine (T3); production is regulated by the hypothalamic-pituitary axis via secretion of thyroid-stimulating hormone (TSH). Circulating thyroid hormone is predominantly bound to thyroglobulin, also produced by the thyroid. Calcitonin is produced and secreted by parafollicular C cells, which are derived from the ultimobranchial bodies.
12.3 Imaging Evaluation
12.3.1 Ultrasound
The initial imaging modality for the thyroid gland is ultrasound (US), performed using a high-resolution linear probe (7–13 MHz). US is useful for evaluating thyroid nodules and cervical nodes, guiding fine-needle aspirate (FNA) biopsies, and performing surveillance for disease progression or recurrence. US can also be used to assess for fetal goiters and to screen for the presence of thyroid nodules in high-risk groups (e.g., history of childhood radiation exposure).
On US, the thyroid parenchyma appears homogeneous with echogenicity greater than the adjacent muscles. The longus colli muscle and trachea can be seen posteriorly to the thyroid gland on sagittal views. On axial views, the common carotid artery and internal jugular vein are present laterally; sternohyoid, sternothyroid, and sternocleidomastoid muscles are present anteriorly (Fig. 12.4).
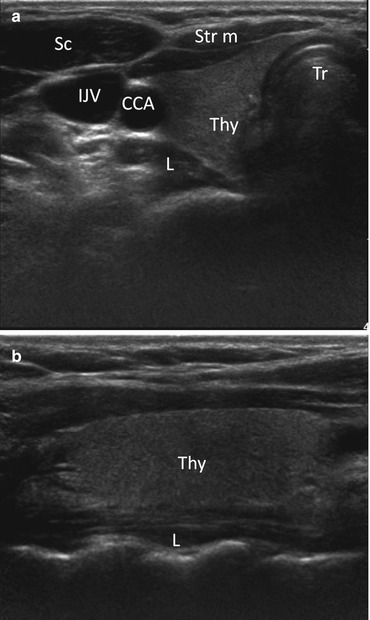

Fig. 12.4
Axial (a) and sagittal (b) grayscale ultrasound images of the right thyroid lobe with adjacent structures. Thy right thyroid lobe, Tr trachea, CCA common carotid artery, IJV internal jugular vein, L longus coli muscle, Sc sternocleidomastoid muscle, Str m strap muscle
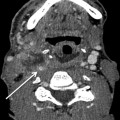
Ultrasonography findings suspicious for thyroid cancer include a taller-than-wide shape, spiculated or microlobulated margins, marked hypoechogenicity, and micro- and macrocalcifications. A combination of size and suspicious US features is more predictive of malignancy than size alone. When FNA biopsy of a nodule is clinically appropriate, US guidance is recommended for nodules that are nonpalpable, pre- dominantly solid, and/or located posteriorly in the thyroid lobe. US-guided FNA is done using narrow-gauge needles (22G or 25G), with or without local anesthesia, and adequate samples can be obtained in 90–97 % of solid nodules (Fig. 12.5). The American Thyroid Association and Society of Radiologists in Ultrasound recommendations are summarized in Box 12.1.
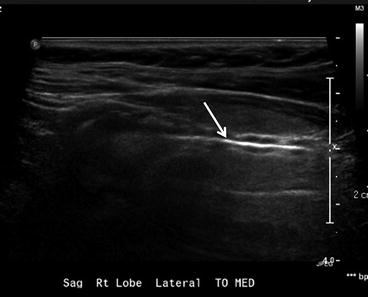

Fig. 12.5
Fine-needle aspiration of thyroid lesion. Sagittal ultrasound image showing the entire needle (arrow) as a hyperechoic structure. This appearance is seen using the parallel needle technique, with both the needle and ultrasound transducer in a plane parallel to each other
12.3.1.1 Box 12.1. Summary of FNA Recommendations for Thyroid Nodules Based on Clinical and Sonographic Criteria
Sonographic and clinical features | American Thyroid Association | Society of Radiologists in Ultrasound (≥1 cm) |
|---|---|---|
High-risk historya and suspicious sonographic featuresb | Strongly recommend FNA if >5 mm | None |
Substantial growth | None | Consider FNA |
Cystic nodule | FNA not indicated | FNA not indicated |
Solid composition | Recommend FNA if >1 cm and hypoechoic | Strongly recommend FNA if ≥1.5 cm |
Solid and cystic | Recommend FNA if >2 cm | Consider FNA if ≥2 cm |
Coarse calcification | None | Strongly recommend FNA if ≥1.5 cm |
Microcalcifications | Recommend FNA if >1 cm | Strongly recommend FNA if ≥1 cm |
12.3.2 Radionuclide Scintigraphy and PET
In the past, radionuclide imaging was used to assess the functionality of all thyroid nodules as a predictor of malignancy (hot nodules are rarely malignant). Given the effectiveness of thyroid US and FNA to clearly delineate malignant nodules, nuclear thyroid scans are currently used to distinguish between various causes of hyperfunctioning thyroid (Graves’ disease, toxic multinodular goiter, autonomous adenoma) and in the workup of ectopic thyroid tissue. Whole body imaging using iodine-123 (123I) or iodine-131 (131I) is used to monitor post-thyroidectomy patients for recurrence or metastasis and to assess the response to therapy. 123I is the imaging agent of choice for benign thyroid processes due to its short half-life (13 h) and lower energy (159 keV). Because of its longer half-life (8 days) and higher energy (364 keV), 131I is the radionuclide of choice for treatment of Graves’ disease, toxic multinodular goiter, and well differentiated thyroid cancers (papillary and follicular thyroid cancers). 131I is also used for posttreatment surveillance of well-differentiated thyroid cancer. Medullary and anaplastic thyroid carcinomas do not take up radioiodine, but can be evaluated by either technetium-99 m (99mTc) sestamibi imaging or fluorine-18 deoxyglucose (FDG) PET-CT, which are discussed below. 99mTc pertechnetate is the preferred thyroid imaging agent in children due to its low radiation dose, short half-life (6 h), and high count rate.
A normal thyroid gland shows homogeneous radiotracer uptake with areas of increased uptake in the region of greater gland thickness. The pyramidal lobe can be seen as linear uptake extending superiorly from the isthmus in cases of hyperthyroidism (Fig. 12.6). Most cold nodules represent benign lesions such as cyst, adenomas, or colloid nodules; the incidence of cancer in a cold nodule is 15–20 %; thus, they warrant further assessment with US. If a cold nodule is solid on US, biopsy is recommended. Almost all hot nodules represent benign hyperfunctioning adenomas. Given the limited diagnostic information and the frequent need for follow-up US, iodine uptake imaging is no longer considered the preferred initial evaluation for a thyroid nodule.
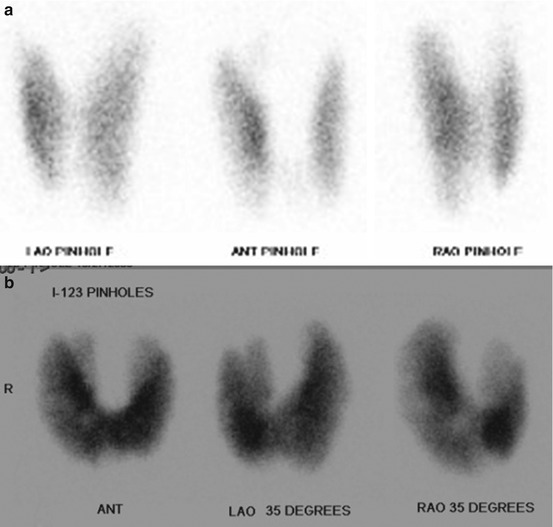

Fig. 12.6
Normal I-123 thyroid scan (a) with uniform uptake of radiotracer by the thyroid gland. Iodine uptake equals 10.3 % at 4 h and 21.2 % at 24 h. Values less than 18 % at 4 h and less than 35 % at 24 h are normal. Abnormal I-123 scan (b) in a hyperthyroid patient shows a diffusely enlarged gland with an identifiable pyramidal lobe. This patient had an iodine uptake of 85.7 % at 3 h
PET-CT is performed using 18F-FDG and is useful in detecting recurrent thyroid cancers in the setting of negative radioiodine scans. The sensitivity of PET-CT is more than 90 % in the setting of elevated thyroglobulin and negative 131I scan after thyroidectomy. Due to their low 18F-FDG uptake, PET-CT is limited in its ability to evaluate poorly differentiated and aggressive thyroid tumors, except to identify distant metastasis.
12.3.3 CT and MRI
The primary role of cross-sectional imaging (CT or MRI) is for presurgical evaluation for extra thyroidal extension and regional metastasis of thyroid cancers. This includes evaluation for invasion of the trachea, esophagus, larynx, recurrent laryngeal nerve, infrahyoid strap muscles, subcutaneous soft tissue, mediastinal and great vessels, and prevertebral fascia. While US can evaluate for cervical lymphadenopathy, retropharyngeal and mediastinal nodes are difficult to access with US and are better evaluated by CT or MRI. Contrast-enhanced CT is discouraged prior to surgery as it can impact the uptake of 131I used for postoperative thyroid ablation. When iodinated contrast agents have been used, 131I treatment is typically withheld for 6 weeks and urine iodine measurements may be necessary to ensure uptake. Thus, non-contrast CT or contrast-enhanced MRI is preferred preoperatively; of the two, MR provides better soft tissue resolution.
12.3.4 Imaging Incidentalomas
Thyroid incidentalomas are nonpalpable thyroid nodules that are detected during imaging procedures ordered for other reasons. Nonpalpable nodules have approximately the same risk of malignancy as palpable nodules. However, 18F-FDG-avid nodules discovered on PET scans have a higher risk of malignancy relative to those discovered on CT or MRI. The American Thyroid Association (ATA) and Society of Radiologists in Ultrasound (SRUS) guidelines, summarized in Box 12.1, are based on clinical risk factors and ultrasound evaluation. Although there are no uniform guidelines for non-US (CT, MRI, or PET)-detected incidentalomas, a practical approach includes further work based on imaging findings and clinical history. This has been summarized in Box 12.2.
12.3.4.1 Box 12.2. Practical Approach for Incidental CT-, MRI-, or PET-Detected Thyroid Nodules
Malignancy risk category | Imaging characteristics | Workup recommendations |
|---|---|---|
Low risk | <1 cm sized nodule with absence of risk factors | Description in body and/or impression of radiology report |
High risk | >1 cm size nodule | Ultrasound for further characterization and possibly FNA (dependent on the additional workup) |
High-riska clinical history, younger patients <20 years, or males <35 years or females 20–35 years | ||
Concerning imaging features (associated lymphadenopathy, extrathyroid spread of lesion, vocal cord palsy, distant metastasis, PET avid lesion, etc.) |
12.4 Pathology
From an imaging perspective, the diseases of the thyroid gland can be divided into congenital/developmental, diffuse, and focal diseases. The congenital diseases include thyroglossal duct cysts (TGDCs) and ectopic thyroid. The diffuse disease includes goiter, Graves’ disease, and multinodular goiter, along with various forms of thyroiditis. The focal diseases consist of thyroid nodules, which are broadly divided into colloid nodules, adenomatous nodules, adenoma, and tumors.
12.4.1 Congenital/Developmental Diseases
12.4.1.1 Thyroglossal Duct Cyst
TGDCs represent thyroglossal duct remnants anywhere between the foramen cecum (tongue base) and the thyroid gland (Fig. 12.3); they are among the most common congenital anomalies in the head and neck. TGDCs are usually midline at or above the hyoid and paramidline below. On CT, these are thin-walled, uni- or multilocular cystic masses and may enhance if infected (Fig. 12.7). Approximately 50 % occur at the level of the hyoid, either anterior to hyoid or extending into the preepiglottic space. About 25 % are suprahyoid, within the tongue base or floor of mouth, and about 25 % are infrahyoid, embedded within the strap muscles. TGDCs are most effectively treated with the Sistrunk procedure, which, in addition to the cyst, resects the entire tract and central hyoid bone to reduce recurrence. Rarely, these can have associated papillary thyroid cancers, noted as an enhancing solid nodule within the cyst (Fig. 12.8).
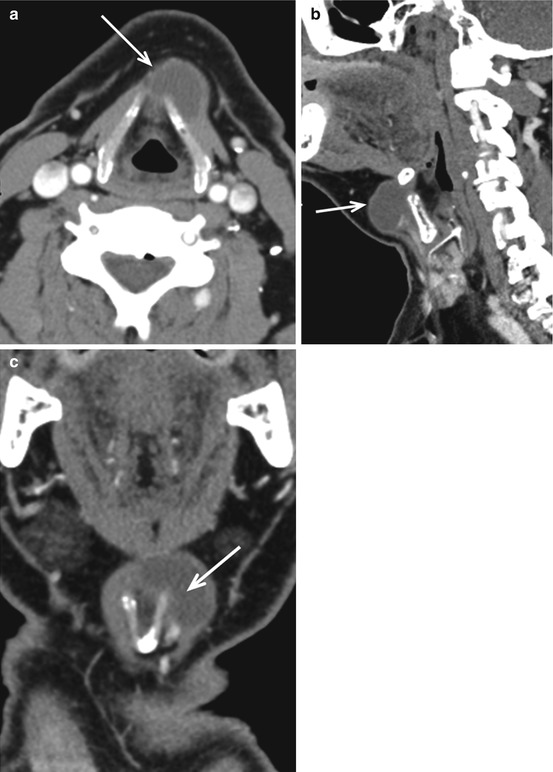
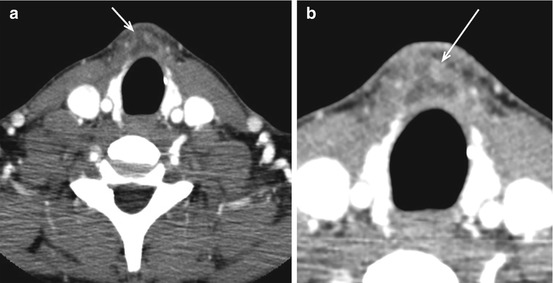

Fig. 12.7
Infrahyoid thyroglossal duct cyst. Rim-enhancing cystic lesion (white arrow in a, b and c), in the left paramedian location, wrapping around the left thyroid lamina and embedded within the strap muscles on axial (a), sagittal (b) and coronal (c) scans. Unlike suprahyoid thyroglossal duct cysts which are usually midline, infrahyoid cysts are paramedian in location. Enhancing solid nodules or thick calcifications within the cyst suggest an associated thyroid carcinoma

Fig. 12.8
Papillary carcinoma in thyroglossal duct cyst. Heterogeneously enhancing lesion in the pretracheal location (arrow in a) with a more nodular focus within it (arrow in b). FNA of the lesion came back as a papillary carcinoma arising within a thyroglossal duct cyst
12.4.1.2 Ectopic and Lingual Thyroid
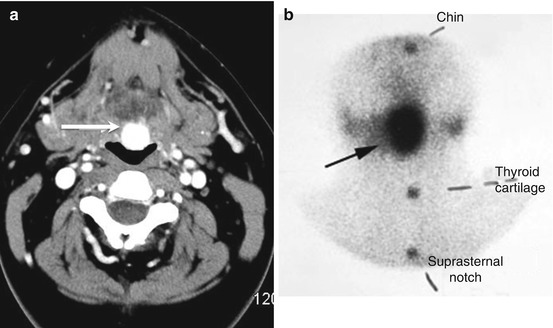
Ectopic thyroid tissue may be found anywhere (Fig. 12.9) within the central neck along the course of the thyroglossal duct (Fig. 12.3). While lingual thyroid represents the most common location (90 %), ectopic thyroid tissue has been identified as far inferiorly as the mediastinum. Lingual thyroid tissue is usually asymptomatic but may present with irritative or obstructive symptoms. Due to its iodine content, it appears as a hyperdense midline mass within the tongue on non-contrast CT (Fig. 12.10). The presence of thyroid in the normal location should be verified as the ectopic thyroid tissue may represent the only functioning thyroid tissue (75 %). Rarely, these may undergo goitrous or malignant transformation (3 %). Rarely, two separate ectopic thyroid tissues can be identified (Fig. 12.11).
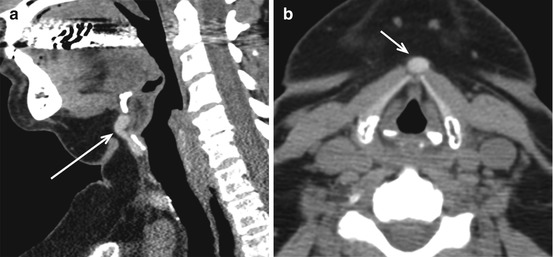

Fig. 12.9
Ectopic thyroid. Sagittal (a) and axial (b) non-enhanced CT images demonstrate a well-defined hyperdense (arrows) lesion just below the hyoid, representing an ectopic thyroid tissue. The presence of iodine in thyroid tissue makes them hyperdense on non-contrast CT scans, making it an easy diagnosis