Radiologists very frequently encounter incidental findings related to the thyroid gland. Given increases in imaging use over the past several decades, thyroid incidentalomas are increasingly encountered in clinical practice, and it is important for radiologists to be aware of recent developments with respect to workup and diagnosis of incidental thyroid abnormalities. Recent reporting and management guidelines, such as those from the American College of Radiology and American Thyroid Association, are reviewed along with applicable evidence in the literature. Trending topics, such as artificial intelligence approaches to guide thyroid incidentaloma workup, are also discussed.
Key points
- •
Thyroid incidentalomas are very common and can be initially detected on computed tomography, MR, ultrasound, PET, or other modalities.
- •
Most thyroid nodules are benign, and most malignant nodules are papillary carcinomas with a favorable prognosis.
- •
Appropriateness of dedicated sonographic evaluation of incidental thyroid nodules depends on nodule size, presence of aggressive imaging features, patient age, and absence of comorbidities that limit life expectancy.
- •
Thyroid ultrasound permits stratification of malignancy risk of thyroid incidentalomas and can guide decisions for biopsy or follow-up with imaging.
Introduction
In routine clinical practice, radiologists are very likely to encounter incidental thyroid abnormalities during interpretation of imaging studies of the neck, chest, or spine. Occasionally, a diffuse thyroid abnormality, such as goiter, may be incidentally encountered ( Fig. 1 ), particularly in areas with iodine deficiency, but this article will primarily discuss recent literature relevant to the thyroid incidentaloma or incidental thyroid nodule (ITN), a term that refers to an asymptomatic thyroid nodule identified on imaging studies not specifically intended for assessment of thyroid pathologic conditions. Although incidental detection of an ITN often leads to further evaluation to exclude or diagnose malignancy, fortunately, most ITNs are benign, and most thyroid cancers are papillary thyroid carcinomas, which generally have an excellent prognosis. Familiarity with existing guidelines and evidence-based recommendations regarding ITNs will enable radiologists to more effectively and appropriately communicate the significance of incidentally detected abnormalities to patients and referring providers.

Prevalence of thyroid incidentalomas
A familiarity with ITNs is crucial because they are exceedingly common in clinical and radiology practice, with reported prevalence varying by the examined population and assessment technique. Based on postmortem examinations of thyroid glands of asymptomatic subjects, thyroid nodules are present in approximately half the population. In contrast, thyroid palpation detects nodules in roughly a fifth of asymptomatic subjects. In general, thyroid nodule prevalence varies somewhat linearly with age and shows a strong female predominance. , Incidentalomas on imaging are reported at frequencies between that of autopsy and palpation, although one should keep in mind that imaging utilization, which varies with age and gender, can have confounding effects on the prevalence and malignancy risk of ITNs.
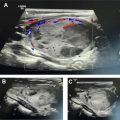
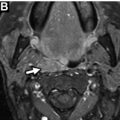
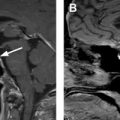
On neck computed tomography (CT) examinations, ITNs have a prevalence of 16% to 18% based on retrospective investigations, , but they are described in clinical radiology reports at a lower rate of 6%. About 1 in 4 contrast-enhanced chest CT examinations have an ITN, and lung cancer screening chest CTs can potentially contribute to incidental detection of thyroid abnormalities. CT represents a very common modality on which ITNs or incidental thyroid cancers are initially detected. Prevalence of ITNs on MR imaging ( Fig. 2 A, B ) is similar to that of CT. However, MR imaging represents a much smaller contribution to incidentaloma detection than CT. , ,

Ultrasound (US) is generally the modality of choice for characterizing ITNs, but US studies performed for unrelated indications, such as assessing neck vasculature or in a screening context, can result in incidental detection of nodules. US-detected thyroid nodules represent a sizable component of ITNs. , , Sonography in randomly selected individuals in a Finnish study detected ITNs in 21% and diffuse abnormalities in 6%. An Italian study examining US examinations in individuals without thyroid disease reported an ITN prevalence of 33%, similar to findings from a large Korean study showing prevalence of thyroid nodules or cysts to be 34% among subjects undergoing thyroid US during routine health evaluations. Prevalence of ITNs as high as 67% on US has been reported.
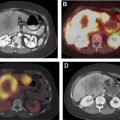
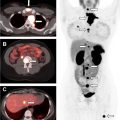
Focal thyroid gland uptake on fluorodeoxyglucose (FDG)-PET ( Fig. 3 ) is detected in only 2% to 3% of oncologic PET studies. , Nonetheless, PET-detected ITNs comprise a substantial portion of imaging-detected incidentalomas , and account for a quarter of thyroid cancers initially detected on imaging. Other modalities may detect incidental thyroid cancers and nodules, such as other nuclear medicine studies (octreotide scans), chest radiography, and echocardiography, , but incidentalomas detected on these modalities are much less common.

An era of incidental findings?
According to the Surveillance, Epidemiology, and End Results (SEER) Program, thyroid cancer incidence is 15.8/100,000 per year, with a mortality of 0.5/100,000 per year Much has been written about the seemingly alarming increase in thyroid cancer incidence of approximately 3-fold over the past 4 decades. , Most of the increased incidence is attributable to more frequent detection of subcentimeter papillary thyroid carcinomas. However, because overall mortality from thyroid cancer has remained relatively stable, several investigators have described this phenomenon as a problem of overdiagnosis. One mechanistic explanation underlying the observed increase in thyroid cancer incidence is the presence of a large reservoir of asymptomatic, indolent thyroid cancers that may never reach clinical attention. The near-ubiquity of clinically occult thyroid cancers is demonstrated in 1 study that found foci of papillary carcinoma, most of which were less than 1 cm, in 36% of consecutive autopsies, indicating a high prevalence of small foci meeting histologic criteria for carcinoma that may never manifest as a clinically apparent cancer.
There are other factors that may contribute to increased detection of incidental findings. Increasing utilization of cross-sectional imaging modalities, including a 10% per year growth in CT imaging use over a similar time period and increased use of point-of-care sonography, because of higher quality and lower cost of US equipment, is thought to contribute to a large portion of the observed increase in thyroid cancer diagnoses. In addition, incidental cancers may also be detected at a greater rate because of rising rates of fine-needle aspiration (FNA) and thyroid surgeries. One study of claims databases from 2006 to 2011 demonstrated annual increases of 16% for thyroid FNAs and 12% for total thyroidectomies in the United States. Therefore, increased imaging detection or increased diagnostic scrutiny could potentially result in higher thyroid cancer incidences over time.
However, some investigators have reported a small yearly change in incidence-based thyroid cancer mortality on the order of 1% per year, raising the possibility of a relatively small superimposed increase in true cancer risk in addition to effects of increased detection. Incidence of clinically symptomatic or palpable cancers has also increased, suggesting that there may be other factors contributing to these thyroid cancer incidence trends. Regardless of the cause of the observed growth in thyroid cancer diagnoses, the relatively indolent course of most incidental thyroid malignancies has led to a growing interest in the radiology community to refine and standardize radiology reporting and management recommendations for this very common incidental finding.
Cancer risk in thyroid incidentalomas
Thyroid Nodule Epidemiology
Most ITNs are benign. Malignancy risks among ITNs have been reported at approximately 12% in patients undergoing US-guided FNA. Estimates of malignancy risk among CT-detected ITNs are similar at 11%. FNA and surgical series tend to overestimate malignancy risk for ITNs because of ascertainment bias, because many low-risk nodules will not undergo FNA or surgery, and therefore, would be underrepresented in the cytopathologic or histopathologic data. A population-based study estimating cancer risk among thousands of patients who underwent US evaluation of ITNs found a malignancy risk of 1.6% for thyroid nodules at least 5 mm. Although this malignancy risk estimate is lower than that obtained in FNA or surgical series, the linking of a cohort of more than 8000 patients with cancer registry data allowed that study to capture cancers detected as late as 6 years after the US evaluation. Therefore, it likely yields a more reliable and unbiased estimate of malignancy risk for incidentalomas, irrespective of decisions to perform FNA at the time of imaging.
What Patient Factors Affect Malignancy Risk and/or Prognosis?
One of the early studies examining ITNs in both CT and US detected an increased malignancy risk for thyroid incidentalomas in patients less than 35 years of age. One later study showed better prediction of malignancy when dichotomizing at an age threshold of 52 years. From age 20 to 60, relative risk of malignancy decreases 2.2% per year. However, the effects of age are complicated by the observation that despite the lower risk of malignancy among elderly patients, thyroid cancers identified in older patients are more likely to demonstrate higher-risk histologies. Nonetheless, existing literature offers some justification for a less aggressive management approach for elderly patients. In 1 study of patients at least 70 years of age who had undergone US and FNA for thyroid nodules, the likelihood of death from thyroid cancer, as assessed during follow-up intervals averaging 4 years, was very low (<1%), and 94% of deaths in this cohort were due to causes unrelated to thyroid disease. Of those who did die from thyroid cancer, all had significant-risk thyroid cancers that were not subtle on imaging and/or cytology and were easily discerned at the time of thyroid nodule evaluation. Other potential factors that may increase risk of malignancy in ITNs include male gender, , radiation exposure in childhood, , and family history of thyroid cancer.
What Nodule Imaging Characteristics Affect Malignancy Risk and/or Prognosis?
Imaging findings for most ITNs on CT or MR imaging do not permit reliable determination of benignity or malignancy, but the presence of some highly suspicious findings, such as aggressive local invasion, suspicious lymphadenopathy, or systemic metastatic disease, can be used to assign higher risk to nodules seen on CT or MR imaging ( Fig. 4 ). Most of the literature quantifying malignancy risk in ITNs is based on ultrasonographic features, but size is 1 property that can be assessed on different modalities. The relationship between nodule size and malignancy risk in the literature is variable, ranging from absent to a modest positive correlation. , A study using population-based SEER data to predict thyroid cancer outcomes as a function of tumor and patient variables using a proportional hazards model found that tumor size only increased mortality when size exceeded 2.5 cm. Another population-based study following all patients who had thyroid nodules examined under US found size greater than 2 cm to be one of 3 sonographic findings significantly associated with cancer risk.

The other 2 sonographic determinants of cancer risk in the above population-based study were microcalcifications and an entirely solid composition. Microcalcifications in a solitary solid thyroid nodule confer a 48% chance of malignancy based on regression analysis of FNA data in 1 study. In a separate multi-institutional series, thyroid nodules with solid composition had a malignancy risk of 13% compared with 4% for mixed solid and cystic nodules. In addition to nodule composition and calcifications, other imaging features in the sonographic literature that affect risk of malignancy in thyroid nodules include echogenicity, shape, and margins. , Sonographic determinants of malignancy have been reviewed in further detail in recent publications. ,
Published guidelines for reporting and evaluating incidental thyroid lesions
Overview of Existing Guidelines
Before efforts by the American College of Radiology (ACR) to adopt a standardized reporting system, there had been high variability among radiologists regarding the reporting of ITNs , and subsequent workup of reported incidentalomas. Reduction in ITN workup can be achieved with minimal risk of missing aggressive cancers by applying varying size thresholds for different levels of estimated malignancy risk. , , , , Several approaches to stratifying malignancy risk on sonography have been proposed, including pattern-based categorization systems proposed by the American Thyroid Association (ATA) and other organizations. , In 2017, the ACR finalized a feature-based grading system, designated ACR Thyroid Imaging Reporting and Data System (ACR TI-RADS), loosely modeled after the Breast Imaging Reporting and Data System for mammography reporting. Most of the discussion in the following subsections focuses primarily on recommendations from the ACR and ATA.
Decision to Pursue Dedicated Thyroid Ultrasonography
ITNs are frequently detected on CT, MR imaging, or PET as nonspecific nodular findings. In general, ultrasonography allows more definitive characterization of these nodules, but the need to detect potential thyroid malignancy must be weighed against the potential harms of pursuing definitive evaluation and treatment of a predominantly indolent and asymptomatic incidental finding. A radionuclide thyroid scan is unnecessary in most patients and is only helpful in the setting of low serum thyroid-stimulating hormone. Often, before pursuing further evaluation, review of prior available imaging may be helpful to determine the presence or absence of the nodule on a prior imaging study, even if not explicitly mentioned in the imaging report, and interval change can provide critical information regarding the malignancy risk of the nodule. For instance, a nodule with long-term stability is unlikely to represent a malignancy, whereas a nodule not present on a scan a year ago or having doubled in size over a short time interval raises concern.
A diagnostic thyroid US examination typically involves a high-resolution sonographic evaluation of the neck in hyperextension with imaging performed in longitudinal and transverse planes and includes assessment of the thyroid gland and cervical lymph nodes. Sonographic findings determine the need for FNA or sonographic follow-up or may reassure against the need for further nodule evaluation, but not all ITNs require dedicated evaluation with sonography. The 2015 ATA guidelines state that dedicated thyroid sonography “should be performed in all patients with a suspected thyroid nodule, nodular goiter, or radiographic abnormality suggesting a thyroid nodule incidentally detected on another imaging study,” but also include a general statement that evaluation with FNA should only be performed in nodules greater than 1 cm along with an acknowledgment that subcentimeter nodules may occasionally warrant workup because of symptoms or lymphadenopathy. The general practice of avoiding workup of most subcentimeter nodules is supported by data showing subcentimeter thyroid carcinomas have a favorable prognosis amenable to nonsurgical management using imaging surveillance.
An ACR white paper published in 2015 provides evidence-based recommendations for communicating workup recommendations for ITNs incidentally detected on radiologic imaging. According to these recommendations, dedicated ultrasonography should be performed on all ITNs that are accompanied by suspicious imaging features, such as evidence of local invasion or lymphadenopathy, regardless of nodule size or patient age. For this purpose, focal radiotracer uptake on a nuclear medicine study is considered a suspicious imaging feature, because focal ITN uptake on PET confers a relatively high risk of malignancy (as high as 50%–60%). , , ,
In otherwise healthy patients without suspicious imaging features, the ACR recommends US for nodules meeting a minimum size threshold of 1 cm in patients younger than 35 years and 1.5 cm in patients 35 years and older. Pursuing sonographic evaluation may not necessarily be warranted in patients with comorbidities that limit life expectancy or increase treatment risks. For patients with such comorbidities, the ACR white paper recommends against further evaluation in the absence of suspicious imaging features, even if nodules exceed the aforementioned size thresholds. For example, in most patients with stage IV lung cancer incidentally noted to have an ITN on staging scans, further workup of the ITN is generally not indicated. Treatment of the more aggressive malignancy is preferable to interrupting therapy to investigate a much less concerning thyroid neoplasm. In an elderly cohort of patients with ITNs undergoing US and FNA described above, close to half had a comorbidity, such as coronary artery disease, or another primary malignancy at the time of nodule evaluation that more than doubles the risk of all-cause mortality, suggesting that even if workup yields a malignant cytologic diagnosis, there may be relatively little benefit on overall survival.
Decision to Perform Fine-Needle Aspiration or Surveillance
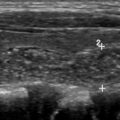
High-resolution thyroid ultrasonography is the test of choice for determining the need for tissue sampling or imaging surveillance. Nodule size can be reliably assessed on US and has been incorporated into both the ATA guidelines and the ACR TI-RADS management recommendations in the form of size thresholds for FNA or surveillance that vary depending on the risk categorization for a given nodule , , ( Table 1 ). In ACR TI-RADS, points are assigned based on sonographic determination of composition (cystic/solid characteristics), dominant echogenicity pattern, shape, margins, and echogenic foci, , , , with sonographic features associated with higher malignancy risk, such as taller-than-wide shape or microcalcifications ( Fig. 5 ), awarded more points. These points are summed for the nodule of interest to determine placement in one of 5 risk categories ( Table 2 ). Validation of the ACR TI-RADS criteria has been performed in a multi-institutional study of more than 3000 nodules that found that the vast majority (86%) of nodules showed empiric malignancy risks within 1% of the specified ACR TI-RADS risk thresholds. One of the main differences between ACR TI-RADS and other systems is that it uses a set of imaging characteristics that can be independently assessed, whereas the ATA and several other systems use a pattern-based approach. Both the ATA and ACR TI-RADS systems recommend against FNA for nodules falling under their most benign risk category ( Fig. 2 C, D). At the highest-risk category, both use a 1-cm threshold for recommending FNA. Between these extremes, ACR TI-RADS and ATA differ slightly in the size threshold used, with ACR TI-RADS using higher size thresholds for FNA. In addition, ACR TI-RADS does not recommend FNA of spongiform nodules ( Fig. 6 ), whereas ATA recommends FNA for spongiform nodules above 2 cm.