■
Central Airway Anatomy and Physiology: Essentials for the Radiologist
Anatomy
The trachea is a cartilaginous and fibromuscular conduct extending from the lower border of the larynx (2 cm below the vocal cords, at the level of spinal C6) to the carina (at the level of spinal T5), where it bifurcates into the main stem bronchi. The trachea ranges from 10 to 12 cm in length, with an extrathoracic portion above the sternal manubrium that ranges from 2 to 4 cm. Sagittal and coronal tracheal diameters vary according to patient height, weight, age, and sex. The sagittal diameter ranges from 13 to 27 mm in males and 10 to 23 mm in females, and the coronal diameter ranges from 13 to 25 mm and 10 to 21 mm, respectively.
C -shaped cartilaginous rings, from 16 to 22 rings, define the anterior and lateral contours of the trachea. Cartilaginous rings reinforce the tracheal wall and maintain lumen patency during expiration. These cartilaginous rings tend to calcify with advanced age, particularly in women, and in patients on long-standing warfarin therapy. The posterior portion of the tracheal wall, also known as pars membranacea, is a thin fibromuscular membrane closely connected to the anterior esophageal wall. Constantly in motion, the pars membranacea assumes a convex and concave shape at end-inspiration and end-expiration, respectively, and contains a small amount of smooth muscle, often referred to as tracheal muscle . The tracheal muscle has an inner circular muscle layer and an external longitudinal, layer. These layers are similar to those of the esophagus, having a common embryologic origin, although constrictor capacity is limited. Main stem and lobar bronchi also have cartilaginous and fibromuscular elements, but the cartilaginous support varies while moving distally toward the lung periphery, with more irregularly shaped cartilage plates at more peripheral levels.
Branches of the inferior thyroid arteries and extreme right intercostal artery provide blood to the trachea superiorly, whereas blood is provided inferiorly by branches of the bronchial arteries and third intercostal artery. Branches of the vagus and recurrent laryngeal nerves and sympathetic trunks innervate the trachea, and the parasympathetic nervous system is the main pathway in control of airway smooth muscle and vessels. These nerves cause bronchoconstriction, mucus secretion, and bronchial vasodilation.
The trachea is lined with pseudostratified, columnar, ciliated epithelium containing goblet cells, which produce mucins, the main component of mucus, to moisten and protect the airways. Mucus and cilia execute the mucociliary function—that is, the excretion of inhaled foreign particles that are pushed toward the larynx and then the pharynx, where they can be swallowed or expelled as phlegm.
Physiology
Tracheal anatomy is related to its functions. First, because the trachea is an airway conductor, its structure must be elastic so it can sustain the conformational changes occurring during the breathing cycle. The trachea elongates at end-inspiration with a three-dimensional (3D) change in size and location, and elastic recoil of the trachea restores its original length and position with the C -shaped cartilages, preventing collapse during expiration. In the general population, there is a large variability in the range of central airways collapse, with studies showing up to 78% collapse in healthy subjects. More detailed description of central airways collapse assessment is given in the following sections. Second, the ciliated mucous membrane of the trachea plays a crucial role in tracheobronchial clearance, the mechanism for clearing the airways. This mechanism is assisted by the coughing reflex, which pushes out the mucus eventually expelled as phlegm through the mouth or swallowed down the esophagus. Mucus film also represents the first barrier of the immune system because it contains glycoproteins, immunoglobulins, albumin, lipids, enzymes, antienzymes, antibacterials, cell products, and mediators. Finally, the central airways contribute to the air-conditioning capacity of the upper respiratory tract, mainly carried out in the nasal cavity. The capacity to heat and humidify the air mainly occurs during normal mouth breathing during exercise and during pathologic mouth breathing resulting from nasal obstructions, such as the flu.
■
Appropriateness of Imaging Modalities: Determining Which Technique Should Be Used
Chest X-Ray
A chest x-ray (CXR) represents a fast, low-cost, and low-radiation modality that offers a preliminary assessment of central airway pathology; however, the purpose is mainly to exclude parenchymal or other sources of clinical symptoms.
In a CXR, the trachea appears as a flipped Y structure that is darker compared to the surrounding mediastinal tissue due to the air content. Well-defined tracheal lines, called paratracheal stripes , delimit the tracheal contours and are crucial for the assessment of central airways. The right paratracheal stripe is normally seen in the frontal CXR as a line that extends from the level of the clavicles to the right tracheobronchial angle at the level of the azygos arch. This stripe is easily identified by the presence of air on both sides (lung and trachea lumen) and consists of the right tracheal wall, adjacent pleural surfaces, and mediastinal fat. The paratracheal stripe has a thickness of 3 to 4 mm; thickening can be an expression of a different pathology, such as lipomatosis, paratracheal lymphadenopathy, thyroid, parathyroid, or tracheal malignancy, and pleural effusion. Similarly, the left paratracheal stripe represents the left trachea wall, the pleural surface between the left upper lobe and the adjacent mediastinal fat, and is seen less frequently in the frontal CXR than the right paratracheal stripe.
Computed Tomography
CT is the most accurate noninvasive modality for assessing not only the airways, but also the entire thorax. CT provides better characterization of CXR abnormalities, such as abnormal tracheal contours, atelectasis, or diffuse parenchymal disease, that can involve the airways. Nowadays, CT is also frequently performed in patients before interventional bronchoscopic procedures to obtain precise preprocedural anatomic correlation and biopsy guidance of peripheral lung lesions. Finally, CT is routinely used in the follow-up of patients undergoing medical and surgical treatment of airway disease. The only major limitation of CT is radiation exposure, which can be problematic, especially in pediatric patients, who are more sensitive to radiation than adults. This problem has been partially overcome by the new generation of CT scanners, which use automatic exposure control devices that can reduce the dose to less than 3 mSv.
Potential Problem: How to Design and Review Appropriate CT Airway Imaging Protocols
Solution.
Routine CT protocol for central airways assessment includes high-resolution axial images, which provide precise information about the size and shape of the airway lumen, presence and location of abnormalities, and mediastinal, hilar, and pulmonary associated abnormalities. Typical scan parameters and postprocessing reconstruction settings are described in Tables 36.1 and 36.2 . Axial images are also indicated to assess direct and indirect signs of airway diseases, such as bronchial wall thickening and bronchiectasis or trapped air. In cases of suspected airway malignancy or infection, IV contrast administration is preferred; otherwise, an unenhanced study would be sufficient.
| Scanner | Multidetector CT |
| kVp, mAs | 110–140, 50–80 |
| Pitch | ≥1 |
| Field of view | Lung apices to bases or to the first or second branching of the main bronchi |
| Acquisition | End-inspiration; end-expiration a with lower kV and mA settings; forced expiration a with lower kV and mA settings |
| POSTPROCESSING RECONSTRUCTION SETTINGS | FEATURES |
|---|---|
| 2D reconstructions | 1 mm thickness—axial, coronal, and sagittal |
| 2D thick MPRs | 1–10 mm thickness, coronal, ± MinIP algorithm |
| 3D surface rendering | Surface rendering of trachea and main bronchi |
| 3D virtual bronchoscopy | Internal surface rendering of the trachea and main bronchi |
However, axial images are usually combined with two-dimensional (2D) and 3D multiplanar reconstructions (MPRs) of the airways. 2D and 3D reconstructed images enhance the identification of subtle and short-segment airway stenosis. Curved MPRs are useful to measure cross-sectional diameters or the area of nonlinear structures (e.g., stenosis located in a bent or kinked trachea). The minimum intensity projection technique is useful for displaying the distribution of air trapping and emphysema in the lung parenchyma. These MPRs are complementary and do not substitute axial images by providing additional information needed for a complete overview of the complex central airway anatomy.
CT assessment of the central airways begins with visualization of the native axial images. Interpretation should begin with assessment of the tracheal and bronchial lumen and walls, followed by the surrounding mediastinal and hilar structures. It is very important to use consistent lung window settings for airway assessment because bronchial wall thickening can vary significantly using different settings. Recommended window settings for lung and mediastinum assessment are as follows: (1) lung setting, and window level (WL), −450 to −700 HU; window width (WW), 1200 to 1500 HU, (2) mediastinal setting, WL, 40 to 50 HU, WW, 350 to 400 HU.
Magnetic Resonance Imaging
In the last two decades, MRI technology has largely improved, allowing for the introduction of MRI into airway imaging. The main advantage of using MRI over CT is the lack of ionizing radiation, which makes MRI valuable for the pediatric population and repeated follow-up imaging. Moreover, MRI offers the possibility to study airway dynamics by cine mode acquisitions.
A second important advantage of MRI over CT is its intrinsic high tissue characterization. By using different tissue weighting (T1, T2, proton density), MRI can distinguish between different tissue compositions, such as fat and water. Finally, MRI allows the assessment of thoracic vessels without the use of contrast material with either bright contrast using a steady-state free precession readout or dark contrast using a single-shot, turbo spin- echo readout with a black blood preparation.
Spatial resolution with MRI is lower than with CT, usually with collections of isotropic voxels ranging from 2 to 5 mm 3 . The most frequently used sequence is 3D gradient echo (GRE) in breath-hold conditions. It can be acquired using a phase array torso or head-neck-spine coil. The latter setting allows for the improvement of spatial resolution with isotropic voxels around 1 mm 3 . The preferred tissue weighting for central airway imaging is proton density, which enhances the signal of the central airways wall, minimizing the brightness of the mediastinal fat. T2 weighting can be obtained with 2D single-shot fast spin-echo or 3D turbo spin-echo techniques. 3D acquisition enables 3D postprocessing techniques similar to those obtained with CT.
Potential Problem: How to Design and Review Appropriate Mr Airway Imaging Protocols
Solution.
For central airway assessment, single-voxel thick (1–10 mm) 2D MPRs are usually displayed simultaneously in the coronal, sagittal, and oblique planes. Thick-slab 2D MPRs can be used with a minimum intensity projection algorithm, a technique that improves the visibility of the airways from the surrounding lung parenchyma by highlighting the lowest attenuation voxels (i.e., air). 2D MPRs improve the detection and assessment of airway stenoses, longitudinal extent of airway stenoses, and complex congenital airway abnormalities. These reconstructions are sometimes combined with curved planar reformats performed along the lumen of an airway, allowing for the depiction of multiple contiguous airway segments on a single plane ( Fig. 36.1 ).
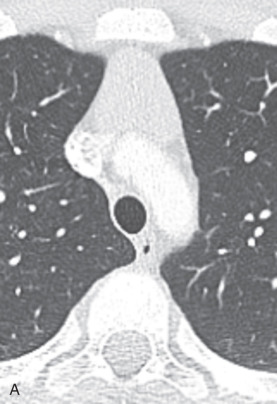



3D reconstruction can be external or internal renderings of the airways. External or surface rendering displays the outer surface of the airways and its relationship with the adjacent mediastinal and lung structures. This technique allows selectively segmenting the central airways, giving a rapid anatomic overview of potential pathologies. 3D surface rendering improves the detection of mild and focal airway stenosis and improves confidence of the findings seen on axial images. Internal 3D rendering, or virtual bronchoscopy, supplies an endoluminal view similar to conventional bronchoscopy. This technique allows visualization of the lumen of central airways until the sixth to seventh order of subdivisions. Virtual bronchoscopy is useful for assessing airway stenosis, guidance of transbronchial biopsy, and foreign body (FB) aspiration. It can be used in situations where conventional bronchoscopy is not desirable (e.g., young children) or feasible (e.g., high-grade obstructing stenosis, beyond which a conventional bronchoscope cannot pass).
The MRI protocol for central airways frequently includes a multiphase dynamic acquisition, performed during specific breathing maneuvers, such as forced expiration, cough, or hyperpnea. These dynamic acquisitions are usually performed to assess tracheobronchomalacia (TBM). Typical sequences used to assess dynamic tracheal collapsibility are 2D axial and sagittal steady-state free precession techniques, 3D GRE multiphase with parallel imaging, or the time-resolved imaging of contrast kinetics (TRICKS) platform. Basic MRI scan parameters for 1.5-T MRI are described in Table 36.3 .
| PARAMETER | BREATH-HOLD ACQUISITION a | DYNAMIC EXPIRATORY |
|---|---|---|
| Sequence | Radiofrequency-spoiled gradient echo sequence (SPGR) b | Time-resolved imaging of contrast kinetics scan (TRICKS) b |
| Repetition time | 1.4 ms | 2.1 ms |
| Echo time | 0.6 ms | 0.8 ms |
| Flip angle | 2–3 degrees | 2–3 degrees |
| Voxel size | 2–3 mm isotropic | 3 mm isotropic |
| Acquisition time | 8–12 s (± parallel imaging) | 48 phases in 19 s |
a End-inspiratory and end-expiratory.
b General Electric brand names; comparable MRI sequences have different names with other vendors.
Central airway assessment using MRI is similar to CT, except that with MRI different tissue weighting provides different information. For example,T1 weighting is normally used for vessel visualization, T2 weighting for bronchi, and PD weighting for lung parenchyma and central airways. Therefore, once the abnormality is detected, it is important to assess signal characteristics with different tissue weighting. Moreover, in MRI, different window settings will influence lumen appearance and size, so it is important to keep a constant window setting (average, maximum intensity projection, minimum intensity projection), especially for the assessment of tracheal collapsibility. However, unlike CT, there are no well-defined window settings for MRI. We recommend using an average algorithm and similar WW and WL between end-inspiration and end-expiration (or dynamic) acquisitions.
Despite these advantages, MRI still has lower spatial and temporal resolution than CT, and MRI is more difficult and time-consuming than CT. Finally, the lack of standard MRI protocols between centers limits the experience with this modality in thoracic imaging, so that MRI is still not routinely used in clinical practice for airway imaging.
Potential Problem: How to Prepare the Patient for Airway CT or MRI
Solution.
Any patient that undergoes airways imaging must be adequately prepared for the breathing maneuvers performed during the scans. Assessment of pathology, such as for TBM, requires dedicated expiratory imaging with both CT and MRI. For CT, there are two main types of acquisitions: (1) paired end-inspiratory and end-expiratory CT; and (2) paired end-inspiratory and dynamic expiratory CT. The first type consists of an acquisition at total lung capacity (TLC) and one at residual volume (RV). In this case, the subjects are coached prior to the scan to reach TLC, RV, and breath-holding for a few seconds. The breath-hold time is longer for MR imaging, up to 8 to 12 seconds. The second type consists of an acquisition at TLC and one during forced expiration, which replicates the peak flow maneuver (forced expiratory volume in 1 second [FEV 1 ]). For this dynamic acquisition, patients are trained to reach TLC and blow all air out as fast as they can. Possible variance of this dynamic acquisition is the coughing maneuver, in which the patient is asked to cough out air from the TLC. The aim of these dynamic maneuvers is to elicit the collapse point of the trachea during expiration. One should stress that the patient should exhale without pursing the lips because doing so artificially increases the intraluminal tracheal pressure, which may prevent the manifestation of subtle TBM.
Prior to the scan, the CT or MRI technologist can help the patient rehearse these breathing maneuvers and, if possible, may be assisted by a dedicated technician. This training is crucial for pediatric patients because it reduces the anxiety related to the MRI investigation, improves the efficiency of the MRI time, and increases the number of children who successfully complete the MRI investigation. More recently, some centers have introduced the use of CT and MRI compatible spirometers which improves the reproducibility of these breathing maneuvers and allows acquisition of the images when the patient is at end-inspiration and end-expiration.
■
Pathology
Classification of central airway diseases is relatively complex. Three main classification systems exist: (1) pathology-based, benign and malignant; (2) cause-based, congenital and acquired; and (3) anatomy-based, local and diffuse. For practical clinical purposes, the anatomic classification will be discussed in this chapter ( Table 36.4 ). In each group, the description follows a frequency order, from the most common to the least common.
| LOCALIZED | DIFFUSE |
|---|---|
| Iatrogenic (postintubation stenosis, posttransplantation stenosis) | Tracheobronchomalacia (classification, congenital or primary form, acquired or secondary form) |
| Traumatic | Saber-sheath trachea |
| Neoplasm (mimickers, benign, primary malignant, secondary malignant) | Excessive dynamic airway collapse |
| Congenital (tracheal bronchus, bronchial atresia) | Sarcoidosis |
| Idiopathic laryngotracheal stenosis | Granulomatosis with polyangiitis (Wegener) |
| Extrinsic compression | Tracheobronchomegaly |
| Broncholithiasis | Relapsing polychondritis |
| Anthracofibrosis | Tracheobronchopathia osteochondroplastica |
| Foreign body | Amyloidosis |
| Infection (fungal, rhinoscleroma, tuberculosis) | Inflammatory bowel disease |
Common Localized Central Airway Diseases
Iatrogenic Stenosis
Iatrogenic stenosis includes postintubation and posttransplant stenosis. Postintubation stenosis represents the most common cause of acquired focal airways stenosis and can occur after endotracheal intubation or tracheostomy tube placement. This type of stenosis was frequent before the introduction of the low-pressure cuff endotracheal tube, which has reduced its prevalence to less than 1%. However, the prevalence of tracheal stenosis in a long-standing tracheostomy tube placement is still around 30%.
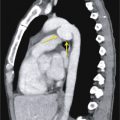
The most frequent site of stenosis after intubation is the subglottic region, at the level of the cuff, whereas posttracheostomy stenosis usually occurs at the stoma site. The most susceptible portions of the trachea are where the mucosa overlies the cartilaginous ring. Post-intubation stenosis is characterized by eccentric or concentric tracheal wall thickening and luminal narrowing, with a craniocaudal length ranging between 1.5 and 2.5 cm. Typical symptoms are dyspnea on exertion, stridor, and wheezing. On CT, the axial image shows focal and circumferential luminal narrowing that can produce the typical hourglass appearance ( Fig. 36.2 ).
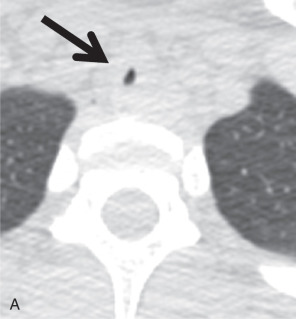



Additionally, posttransplantation bronchial anastomotic stenosis is relatively frequent. In patients for whom lung transplantation is complicated by infection and rejection, the prevalence is even higher. Patients present with symptoms in the first month after transplantation, characterized by a failure to improve and decline of pulmonary function tests (PFTs), especially FEV 1 . On CT, focal narrowing at the anastomotic site can be measured to assess the grade of stenosis, which has a good correlation with the PFT. Treatment of airway stenosis includes bronchoscopic interventions, such as dilation and stenting, and surgical procedures.
Trauma
Airways trauma can be caused by tracheal wall perforation during intubation, direct blunt trauma, or tearing injury of the main stem bronchi at the level of the hilar attachment to the pleura. Regardless of the cause of the trauma, all these lesions tend to heal with stenosis, which can assume the typical hourglass appearance. CT is the method of choice to assess location, morphology, extension, and coexisting complications.
Neoplasm
Central airway neoplasms can be malignant or benign ( Box 36.1 ). Malignant neoplasms are far more common than benign neoplasms and predominate in adults. They are primarily due to local invasion or hematogenous metastatic spread. Benign neoplasms are more common in children and young adults. Central airways tumors are characterized by symptoms of obstruction, such as chronic cough, dyspnea, recurrent infection, and hemoptysis. Dyspnea occurs only in advanced neoplasms, when the airway lumen is substantially narrowed.
Epithelial Neoplasms
Malignant
Squamous cell carcinoma a
a Squamous cell carcinoma and adenoid cystic carcinoma represent 86% of malignant neoplasms of central airways.
Adenocarcinoma
Large cell carcinoma
Neuroendocrine tumors
Carcinoids (typical and atypical)
Large cell neuroendocrine tumor
Small cell carcinoma
Benign
Papilloma
Papillomatosis
Salivary Glands
Malignant
Adenoid cystic carcinoma
Mucoepidermoid carcinoma
Carcinoma
Benign
Adenoma pleomorphic
Adenoma, mucous gland
Myoepithelioma
Oncocytoma
Mesenchymal Neoplasms
Malignant
Soft tissue sarcomas
Chondrosarcoma
Lymphomas
Benign
Lipoma
Fibroma
Fibromatosis
Histiocytoma
Hemangioma
Hemangiopericytoma
Chemodectoma
Leiomyoma
Granular cell tumor
Schwann cell tumor
Chondroma
Chondroblastoma
Secondary Neoplasm
Direct Invasion
Esophagus, thyroid, larynx, lung
Hematogenous Metastasis
Breast, colorectal, renal, ovarian, uterine, testicular, melanomas, and sarcomas
Secondary Malignant Neoplasms
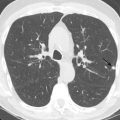
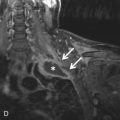
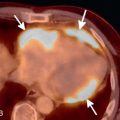
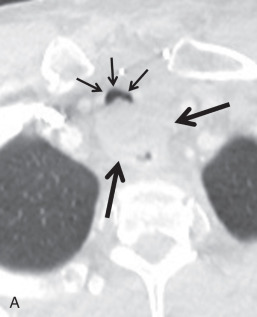
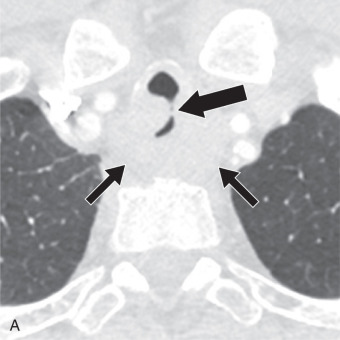
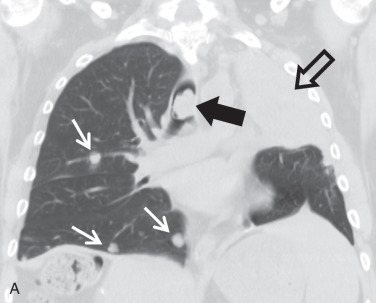
Secondary malignant neoplasms can be the result of local invasion or hematogenous metastatic spread. Local invasion can be from cancers of the thyroid, esophagus, lung, or larynx. Signs of direct tracheal invasion on CT are an endoluminal mass near the primary tumor, cartilage destruction, and fistulization ( Figs. 36.3 and 36.4 ). Tumors that frequently metastasize to the central airways are breast, colorectal, renal, lung, ovary, thyroid, uterine, testicular, melanoma, and sarcoma. On CT, hematogenous metastases appear as endoluminal nodules or eccentric thickening of the airway wall, with soft tissue density and contrast enhancement ( Fig. 36.5 ).






Primary Malignant Neoplasms
Squamous cell carcinoma (SCC) is the most frequent primary neoplasm of the trachea, with a predominance in males (M:F, 2–4 : 1) in the age range of 50 to 60 years. It is highly associated with smoking. Up to 40% of patients with SCC of the trachea develop other smoking-related cancers, such as head and neck or lung cancer. SCC tends to extend locally. When it extends to the esophagus, a tracheoesophageal fistula (TEF) is frequent. CT findings of SCC include a polypoid intraluminal mass, with soft tissue density and irregular and lobulated borders. SCC shows high FDG avidity on PET imaging.
Adenoid cystic carcinoma (ACC) is the second most common malignant neoplasm and originates from the salivary glands. There is no association with smoking habit or sex predominance. The age of occurrence is earlier than that for SCC, usually in the fourth decade of life. ACC appears as an intraluminal focal mass, mostly located in the trachea and main bronchi, with circumferential wall thickening and infiltrative growth along the submucosa. ACC has a slower growth rate and a lesser tendency to invade locally than SCC. CT shows a well-defined soft tissue mass infiltrating the airway wall and, in the late stages, the surrounding mediastinal fat. Circumferential wall thickening can produce localized stenosis, which is better appreciated in the coronal and sagittal MPR images. PET-CT shows high FDG-avidity in ACC.
A third malignant neoplasm is mucoepidermoid carcinoma (MEC). MEC arises from the minor salivary glands lining the tracheobronchial tree. It typically occurs in patients younger than 40 years. MEC is usually located in more distal airways compared to SCC and ACC, commonly involving the lobar and segmental bronchi. The CT appearance of MEC is an oval or lobulated endobronchial mass, with mild contrast enhancement (differential diagnosis with carcinoid).
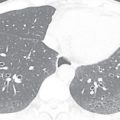
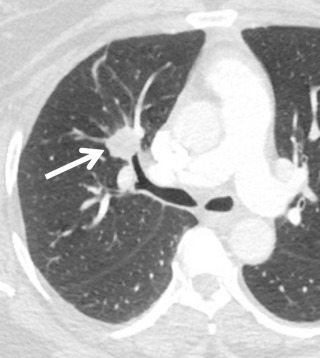
Carcinoid tumors are rare neuroendocrine neoplasms that range from low-grade (typical) to intermediate-grade (atypical) to high-grade small cell carcinomas. Like MEC, carcinoid neoplasms affect patients in their 40s. Carcinoid is the most frequent bronchial tumor in childhood and may produce hormones and neuroamines (e.g., adrenocorticotropic hormone, serotonin, somatostatin). Symptoms related to airway obstruction, such as chronic cough, recurrent infection, and hemoptysis, are more frequent than symptoms produced by hormone secretion. On CT, carcinoid appears as a smooth rounded intraluminal or lobulated mass that may contain calcifications ( Fig. 36.6 ). Carcinoid tumors may have low FDG avidity on a PET-CT scan. Other imaging modalities include somatostatin receptor scintigraphy and Octreoscan, a nuclear medicine technique that uses a somatostatin-radiolabeled analogue (octreotide).
Benign Neoplasms
Benign neoplasms are less frequent than malignant ones, accounting for less than 2% of all lung neoplasms. On CT, benign tumors appear as masses that are within the tracheobronchial lumen and do not show local invasion or distant metastases. Some of these tumors have typical features that help their characterization on CT ( Table 36.5 ).
| Feature | Tumor Type |
|---|---|
| Fat-contacting lesions | Lipoma, hamartochondroma |
| Intralesional calcifications | Hamarthoma, chondroma, carcinoid |
| Fat and calcifications contacting lesions | Hamartoma |
| High contrast enhancing tumors | Carcinoid, hemangioma, fibroma and glomus tumor |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree