Recent developments in cancer genomics have allowed for comprehensive genomic profiling of tumors, thereby allowing oncologists to provide targeted therapies for patients with advanced cancers. This has made it even more important to obtain adequate core biopsies of lesions targeted for biopsies. While most lesions can be biopsied safely percutaneously using CT and ultrasound guidance, some lesions do not have a safe percutaneous window, thereby necessitating alternative biopsy techniques, such as transvenous biopsies, to aid in diagnosis and treatment. This review explores the use and technique of the transvenous approach for targeting small abdominal and pelvic masses using intravascular ultrasound guidance.
Introduction
Recent developments in cancer genomics have allowed for comprehensive genomic profiling of tumors, thereby allowing oncologists to provide targeted therapies for patients with advanced cancers. Additionally, with an increase in the incidence of cancer globally, it has become even more important to obtain adequate tissue samples when performing biopsies. Unfortunately, not all lesions have a safe percutaneous window to proceed with a percutaneous biopsy, posing a grave diagnostic challenge.
While nontargeted solid organ biopsies such as renal and liver biopsies have been safely performed using the transvenous approach, recent advances in intra-vascular imaging over the last decade have allowed us to safely and accurately biopsy small abdominal and pelvic lesions precisely via transvenous approach using side firing Intravenos Ultrasound (IVUS) also known as Intra-Cardiac Echo (ICE), lesions which otherwise may not have a safe percutaneous window.
Recent studies have shown that transvenous targeted biopsies are safe to perform and have diagnostic accuracy (greater than 90%) comparable to targeted percutaneous biopsies performed using CT guidance. , In this review, we will discuss the patient selection criteria, technique, and outcomes of transvenous biopsies as a problem-solving tool.
Patient selection
All patients requiring a biopsy are evaluated in the preprocedural setting, either as an outpatient in the Interventional Radiology (IR) clinic or in the inpatient setting where the procedure benefits, risks, and alternatives are discussed. Many of the patients have already undergone detailed cross-sectional imaging such as a CT scan or MRI of the abdomen or pelvis. In case the patient has not undergone cross-sectional imaging, authors recommend obtaining a CT Abdomen Pelvis with intravenous and oral contrast (if the patient’s renal function allows).
A careful and detailed evaluation of the biopsy target is performed to assess if the lesion can be biopsied percutaneously before proceeding via a transvenous approach, given the widespread availability of the expertise to perform biopsies percutaneously. However, often, there is no safe percutaneous window due to overlying viscera (such as bowel, liver, kidneys, or adrenal glands), osseous structures, or major abdominopelvic vessels. During imaging review, the anatomic relationship of the index lesion with the inferior vena cava (IVC) or the iliac veins is assessed to see if the lesion can be safely biopsied using a transvenous approach. Lesions within 1 cm of the IVC or iliac veins are ideal, accounting for the needle throw of most commercially available transvenous biopsy kits.
Preprocedure work up
The authors recommend preprocedure work up and obtaining basic labs such as complete blood count (CBC), basic metabolic panel (BMP) and international normalized ratio (INR) per providers institutional policy. The need for the type of sedation – moderate sedation versus general anesthesia can be determined based on patient co-morbidities and operator preference and expertise. Authors recommend holding anticoagulation based on individual institutional guidelines for a high-bleeding risk procedure (such as percutaneous CT guided solid organ or deep nonorgan biopsies).
Technique and equipment
The procedure is performed under direct fluoroscopic guidance with the use of side-firing IVUS (ICE). The patient is brought to the procedure room and placed supine on the table. After following institutional protocol for presurgical checklist completion/time out and induction of anesthesia/sedation, the patient is prepped and draped in a sterile fashion.
Transvenous biopsies can be performed via the Internal Jugular Vein (IJ) or Common Femoral Vein (CFV) access, based on the location of the target lesion and operator preference. The authors prefer to access the right IJ for both the biopsy device and IVUS probe, when possible, to avoid the tortuosity of the iliac veins.
Two separate central venous accesses are obtained using ultrasound guidance and using Seldinger technique. A 10 Fr 40 cm sheath is placed for a biopsy device and an additional 9 Fr vascular sheath is placed using the additional access for the IVUS probe. The authors use commercially available trans-jugular liver biopsy kits such as the TLAB Transjugular liver biopsy kit (Argon Medical, Frisco, TX) or the Liver Access and Biopsy Set (Cook Medical, Bloomington, IN). The authors’ experience is based on using the IJ for both the biopsy and IVUS accesses, however other operators use one central venous access (right IJ) for the biopsy device while obtaining the second central venous access via CFV for IVUS. Having both IJ access allows operators to control both the biopsy device and IVUS probe simultaneously.
When the IVUS probe is inserted from the jugular access, the craniocaudal orientation of the image is automatically synchronized. If the probe is introduced from the femoral access, the craniocaudal orientation will need to be changed to reflect the true anatomical orientation. Mediolateral rotation of the probe will bring into view a cross-sectional plane and the level of the transducer. Identifying the aorta using color doppler can be used as a reference point. Structures towards the right of the aorta can be identified using a clockwise rotation of the probe and vice versa for visualizing the structures towards the left of the aorta.
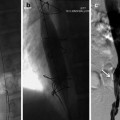
Once both accesses are secured, the target lesion is first identified using IVUS using mesenteric vessels as a landmark and fluoroscopic landmark such as vertebral bodies and disc spaces. Additional imaging modalities, such as cone beam CT can be used intra-procedurally to further confirm that the identified lesion is the intended target lesion.
Once the lesion is identified, the biopsy device is advanced and brought in plane with the lesion under direct IVUS guidance. The biopsy devices have throws ranging from 10 mm to 20 mm. Careful planning is required at this point and sampling distance/throws are adjusted based on the distance of the lesion from the vessel and the size of the lesion.
Technical considerations
Sampling can be repeated as needed using the same steps until an adequate volume of tissue is obtained for the needed histopathologic testing.
Once sampling is complete, the operator can perform venography to confirm no extravasation is present, though this may not be a necessary step in cases where the lesion encases the vein. The needles, wires, catheters, and sheaths can then be removed from the patient. Hemostasis is achieved by manual compression and sterile dressings are applied.
Case examples
Case 1
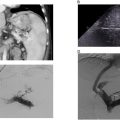
A 35-year-old female with a past medical history notable for systemic lupus erythematosus (SLE) and obstructive uropathy secondary to retroperitoneal fibrosis presents with a growing retroperitoneal soft tissue lesion encasing the inferior vena cava (IVC) and aorta. The lesion had previously been biopsied percutaneously with just FNA (fine needle aspirate); however, the FNA samples were ultimately nondiagnostic.
Due to the vascular involvement, no safe percutaneous window was identified to obtain core biopsy samples ( Fig. 1 ). The patient was subsequently referred to the interventional radiology clinic for discussion of a transvenous biopsy of the mass.