Fig. 12.1
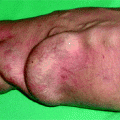
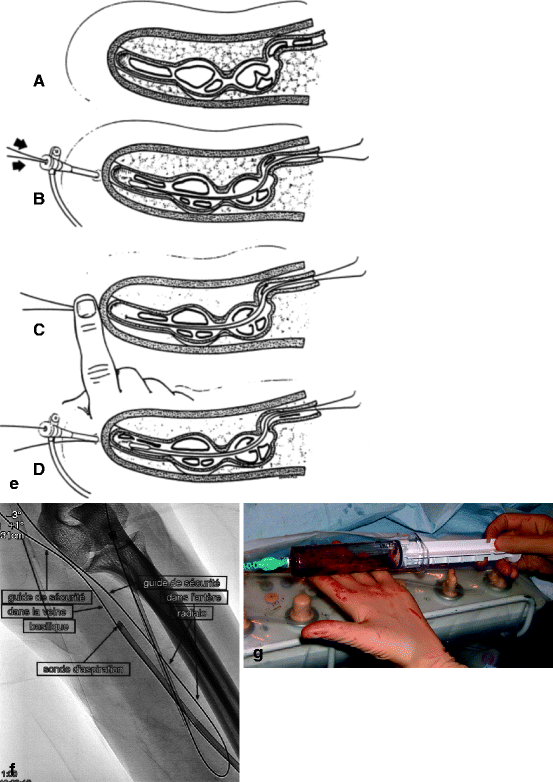
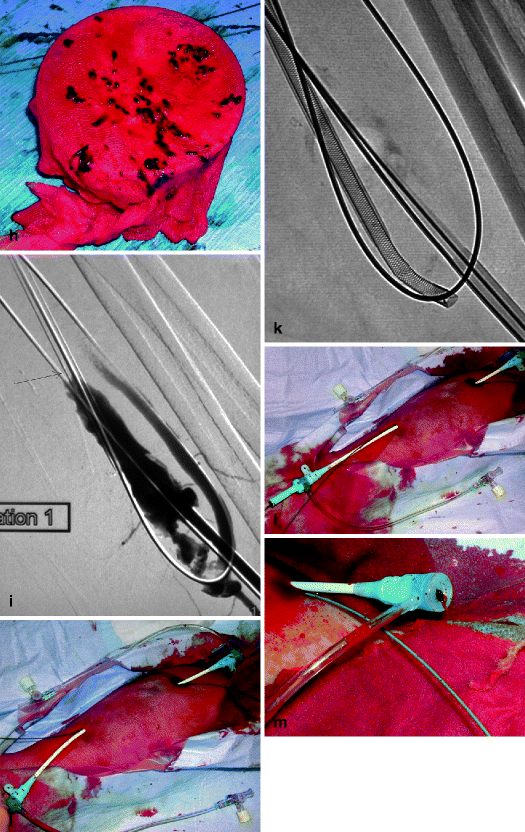
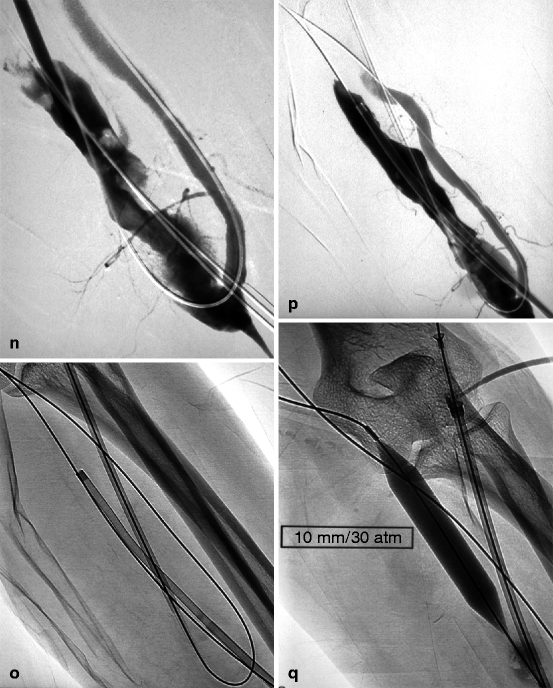
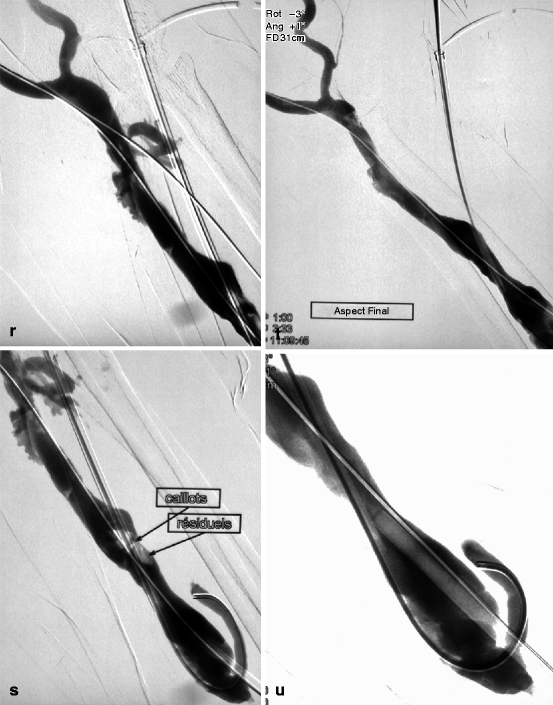
(a) This thrombosed 4-year-old left-transposed (as evidenced by the long surgical scar) radial fistula shows some aneurysmal formation in its cannulation zone, which facilitates blind cannulation. (b) Through an antegrade cannulation of the thrombosed vein, a catheter is advanced over a guidewire into the central veins and then gradually withdrawn as small boluses of iodinated contrast are slowly and gently injected. There are thrombi seen in the arterialized vein segment from just above the elbow while the upper arm cephalic and basilic veins are well opacified and therefore remain patent. (c) Through a retrograde puncture of the thrombosed vein, a catheter is advanced into the anastomosis. Injection of contrast there shows a patent proximal artery perfusing its distal segment. (d) This picture shows the two 8-F introducer-sheaths after placement in opposite direction into the thrombosed vein, with the venous “safety” guidewire shown (arrow) coming directly off the skin puncture but alongside to the introducer-sheath. (e) The four steps of placing a “safety” guidewire. (f) This image shows the aspiration catheter (single arrow “sonde d’aspiration” in French) as it is maneuvered in and out under fluoroscopy parallel to the venous safety guidewire in the more central thrombosed segment ( “guide de securite dans la veine basilique” means “safety guidewire in basilic vein”). The additional legend “guide de securite dans l’artere radiale” means “safety guidewire in radial artery. (g) The 50-mL Vaclok® syringe locked in aspiration mode fills up with a mixture of thrombi and fresh blood. (h) Aspirated thrombi as seen after the aspirated syringe contents are flushed onto a bowl covered with a gauze swab. (i) Having freed the more downstream segment of the vein of thrombi, contrast is seen to collect an outflow stenosis (arrow) which is the cause of the thrombosis. The task is now to aspirate the upstream thrombi near the anastomosis via the “arterial” introducer-sheath. (j) This picture shows the two introducer-sheaths with their “safety” guidewires emerging alongside them. (k) This angiogram shows the aspiration catheter in contact with thrombi near the anastomosis. The arterial “safety” guidewire can be seen across the anastomosis with its tip in the brachial or axillary artery. (l) The “venous” introducer-sheath which can obstruct the in and out movements of the aspiration catheter has been partially withdrawn with its tip now almost at the exit of the cannulation site after repushing its dilator over the wire. (m) A thrombus trapped in the hemostatic valve (arrow) of the introducer-sheath. This can be left alone or the introducer-sheath can be removed over a guidewire, flushed, and then reinserted. (n) The anastomosis has been freed of thrombi, but the fistula is still not circulating as a thrombus has detached after reestablishment of flow and embolized into the stenosis. (o) After reintroducing a “safety” guidewire, an aspiration catheter is advanced through the “venous” introducer-sheath, and the embolized thrombus is aspirated. (p) This angiogram after additional aspiration does not show evidence of residual thrombi. The stenosis can now be dilated. (q) The stenosis is dilated with a 10-mm Conquest® balloon at inflation pressure of 30 atm. (r) Balloon dilation causes moderate venous rupture as evidenced by extravasation of contrast which is successfully controlled with low pressure (2 atm balloon tamponade). (s) Just when you think the procedure is completed, the sudden evidence of a significant mural thrombus (2-arrows “caillots residuels” means “residual clots”) needs to be removed otherwise it can detach later, embolize, and cause access occlusion further downstream. (t) The completion angiogram of the venous outflow is satisfactory after aspiration of the thrombus. (u) Completion angiogram of the anastomosis however shows a remnant thrombotic plaque which should be left alone given it is less likely to detach, embolize downstream, and cause early access rethrombosis
An 18-G cannula and angled tip 0.035-in. hydrophilic guidewire are used for the initial catheterization. Since the access is thrombosed, there is usually no flashback once the cannula correctly enters the access lumen unlike what is usually observed in a functional access. It is therefore not easy to know whether the cannula is actually within the lumen unless puncture is performed under ultrasound guidance. Skin puncture is ideally performed blindly 5–10 mm away from the wall of the vein or graft through a short subcutaneous tunnel, which will facilitate final hemostasis at removal of the introducer-sheath.
Once the cannula is pushed in the direction of the lumen, the needle is withdrawn and the hydrophilic guidewire is gently pushed into the catheter. This part of the procedure should be done under fluoroscopy without any contrast injection. With correct cannula placement, the guidewire should move smoothly into the access lumen without any resistance. The slightest perceived resistance should prompt complete removal of the guidewire, gradual withdrawal of the cannula millimeter by millimeter, and renewed attempts at steering the guidewire through it. This sequence should be repeated with a millimeter withdrawal of the cannula at each cycle until fluoroscopy confirms smooth entry of the guidewire through the cannula into the access lumen or the cannula completely comes out of the vessel.
Cannulation is perhaps one of the most laborious phase of percutaneous thrombectomy particularly in young AVFs which have poorly developed arterialized veins. Once cannula placement within the access lumen is confirmed, it is replaced over the guidewire by a 6- to 9-F introducer-sheath. A 5-F vertebral catheter is then advanced over the guidewire toward the central veins. The guidewire is then removed and 1 mL boluses of contrast are slowly injected to assess if the central veins are patent. At this stage, both heparin and prophylactic antibiotics can be administered via the catheter. The 5-F catheter is then slowly pulled back 1 cm at a time while injecting small boluses of contrast under fluoroscopy to locate the apex or central extension of the thrombosis, which is also the site where thromboaspiration should commence (Figs. 12.1b and 12.2a).
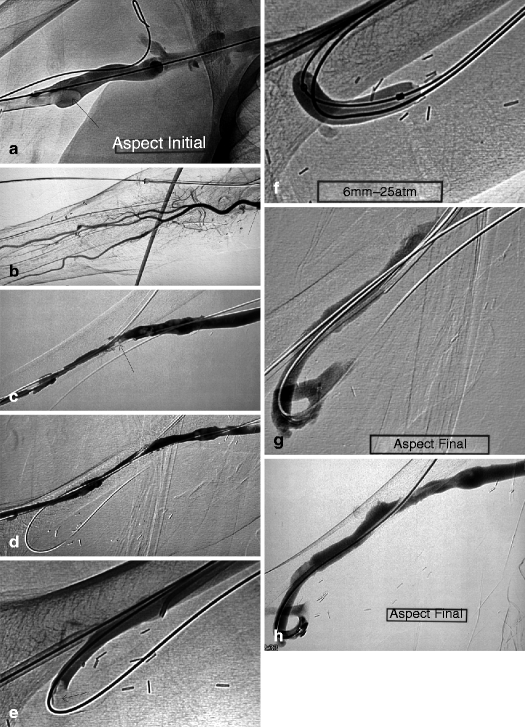
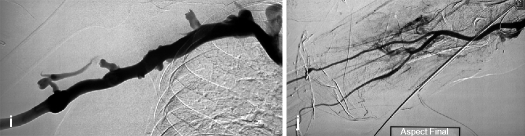


Fig. 12.2
(a) Thrombosis in this transposed right brachial–basilic fistula extends up to the upper third of the upper arm (arrow) where a stenosis is situated which usually develops in superficialized segments. (b) A catheter is advanced across the anastomosis (arrow) into the brachial artery after a retrograde puncture of the thrombosed vein. The angiogram also shows that the three forearm arteries are patent and look normal. An arterial stump indicating a failed attempt at creating a forearm radial fistula can also be seen (double arrow). (c) After a first series of aspiration through the “venous” introducer-sheath, a residual thrombus can be seen at the superficialized basilic vein outflow stenosis (arrow). The guidewire seen projecting outside the lumen of the opacified arterialized vein is the “safety” wire, which traverses the anastomosis, and its tip is in the axillary or subclavian artery. (d) All the residual thrombi have been aspirated, and the outflow stenosis does not appear that severe as it has been pre-dilated by the in and out mechanical action of the aspiration catheter (a technique first described in the 1960s by Charles Dotter, the father of interventional radiology who used rigid catheters to dilate stenoses). (e) The focus is now on the anastomotic segment which hosts an arterial plug (arrow) extremely resistant to aspiration. The acute anastomotic angle does not favor the use of the aspiration catheters. (f) Balloon dilation is eventually used instead to dislodge the arterial plug. Balloon size is kept at 6 mm as is the case with all dilations of anastomotic stenoses in the upper arm accesses. (g) This balloon dilation cum Fogarty catheter thrombectomy seems to have worked as the arterial plug is no longer there (probably embolized to the lungs). (h, i) Completion angiogram of the arterialized vein inclusive of the central veins shows a patent access free of thrombi after dilation of the outflow vein stenosis with an 8-mm balloon. (j) Completion angiogram of the arterial circuit shows occlusion of the interosseous artery (arrow) due to an iatrogenic embolus. This is of no major consequence and should therefore be left alone as both the radial and ulnar arteries remain patent and can maintain distal arm perfusion. It would be more harmful to try and remove this arterial thrombus. The anastomotic stump of a previous radial–cephalic fistula is still visible (double arrow)
Sometimes, the guidewire meets some resistance, and it becomes impossible to advance it further through the venous outflow. The nature of the obstruction should be verified by slowly and cautiously injecting 1 mL of contrast locally through the catheter. As thrombi do not adherently occupy the entire access lumen, the cautious contrast injection usually shows a tight hairline stenosis, a sudden change of access angulation, or straying in a blocked collateral vein as the cause of the obstruction. Adequate skills at maneuvering the catheter–guidewire pair are required to traverse the obstruction and place the catheter within the central veins. Failure to reach the central veins would place the access at a high risk of rethrombosis no matter how successful thromboaspiration is. A combination of a 5-F vertebral catheter and angled tip steerable 0.035-in. guidewire is the most effective tool to pass the obstruction or maneuver across a thrombosed access. A straight tip guidewire has some advantage over an angled tip guidewire in certain situations as we shall see later. Injecting contrast within thrombi, particularly when they involve upper arm accesses, carries a small risk of embolizing thrombotic fragments into the forearm arteries. This is the reason why the injection pressure should be minimal and volume restricted to no more than 1 mL. Some interventionists prefer removing the thrombi first whenever it becomes impossible to cross the outflow obstruction, and then reattempt traversing the venous outflow stenosis or a big collateral vein that might be opened.
Once the central extension of the thrombus has been ascertained, the 5-F catheter is redeployed into the superior vena cava over the hydrophilic guidewire to enable exchange for a “safety” regular metallic 0.035-in. guidewire, less prone to inadvertent removal. The “safety” guidewire tip should not be positioned in the right atrium or ventricle but either in the inferior or superior vena cava where it is unlikely to cause inadvertent endocardial perforation or trigger arrhythmias.
12.6.3 “Arterial” Access
The overall success of thromboaspiration depends on opening and clearing thrombi from both the venous outflow and arterial inflow. After placement of the “venous” introducer-sheath, the “arterial” introducer-sheath is retrogradely (in direction of the arterial inflow) inserted in the arterialized vein or graft 5–10 cm downstream from the entry point of the “venous” introducer-sheath. The tips of the 2 introducer-sheaths should not intersect or overlap as they can occupy too much of the intraluminal space and obstruct flow. The guidewire–catheter pair is used to navigate toward the arteriovenous anastomosis (Figs. 12.1c and 12.2b). Reaching brachial or upper arm access anastomoses is relatively straightforward, but the same cannot be said of radial or forearm access anastomoses given the higher number of collaterals found in the forearm and the higher likelihood of the guidewire/catheter “straying” into one of them. A “strayed” guidewire/catheter can be redirected into the main access lumen and toward the anastomosis by a simple technique of compressing the main vein 1 cm above the tip of the catheter while cautiously injecting contrast and retrogradely opacifying the direction to take to the anastomosis. This technique is risky when performed in upper arm accesses as thrombi fragments can easily be dislodged and embolize into the forearm arteries. Therefore, as a precaution, some interventionists choose the safer approach of placing a metallic landmark (it can be a blade) over the anastomotic surgical scar with the arm in supination as a reference to guide accurate catheterization of the anastomosis. Anastomotic surgical clips at the wrist, whenever visible on fluoroscopy, can also be used on as a landmark for exact location of the anastomosis and hence help guide a “strayed” guidewire/catheter back into the right direction. In the latter scenario, the arm may need to be placed in a prone or transverse position to obtain a better view of the anastomosis which is found anteriorly, in contrast to collaterals which tend to run posteriorly. Arterial calcification which enhances on fluoroscopy may also serve as an anatomical landmark for the anastomosis.
A straight rather than angled tip hydrophilic guidewire is better suited for maneuvering across peri-anastomotic aneurysmal segments as it is less likely to coil up and thus has a greater chance of reaching the vein or artery further upstream. Peri-anastomotic aneurysms are the source of many difficult or failed catheterizations.
Once the anastomosis is traversed, the guidewire/catheter preferentially migrates into the distal rather than proximal artery. Attempts should nonetheless be made to secure access to the proximal artery and to place a “safety” guidewire preferably within. The golden rule of having a “safety” guidewire throughout and for every thromboaspiration procedure should not be taken lightly by both starters and seasoned interventionists.
Getting a guidewire into the proximal artery of upper arm accesses is usually effortless in view of the larger calibered brachial artery. An angled tip hydrophilic guidewire more or less spontaneously finds its way there.
It is more of a challenge in forearm AVFs where the anastomotic angle is either too acute or the radial artery too small. The particularities and challenges of catheterizing radial–cephalic AVF anastomosis have been discussed in Chap. 10. The 4-F internal mammary catheter is most suited for this task.
Once the 4-F catheter led by a hydrophilic guidewire is in the brachial artery, at the level of the elbow or the upper arm, the guidewire is withdrawn and a control angiography of the forearm arterial supply is performed. It is important at this stage to take note of the anatomic and hemodynamic state of the forearm arteries which shall be used as reference on completion of thromboaspiration in order to assess for the presence of any iatrogenic emboli or spasm, particularly in upper arm accesses. Five milliliter of iodinated contrast diluted by 50 % and an image acquisition setting of one image per second for 20 s are adequate for satisfactory opacification of the distal arteries given that arterial blood flow is low in forearm arteries. On completion of a detailed pre-procedure assessment of distal arterial flow, a regular metallic 0.035-in. J-tip “safety” guidewire is advanced into the brachial artery through the 4-F catheter and the latter is removed.
Failure to catheterize the proximal artery, which can happen sometimes, implies that as a compromise the “safety” guidewire is placed into the distal artery.
12.6.4 Venous Outflow Thromboaspiration
Thromboaspiration or any other thrombectomy technique should only take place after 2 introducer-sheaths have been placed over a guidewire in opposite directions across the access: a “venous” one in the direction of the venous outflow and central veins and an “arterial” one placed toward the afferent artery (Fig. 12.1d). With this prerequisite met, it is now likely that the thrombectomy procedure will be successful.
Manual catheter-directed thromboaspiration is a non-over-the-wire thrombectomy technique, which therefore does carry a risk of venous dissection or false tracking. Over the years, after having experienced a few such complications and technical failures, we have found it useful and cautious to mandatorily place the previously described “safety” guidewires in both venous outflow and arterial inflow, in parallel to the aspiration catheter. The “safety” guidewires are especially indispensable when repeat passes of the aspiration catheter are likely to be difficult in accesses which are tortuous, have acute or sharp angulations, segmental stenoses, and aneurysmal dilatations or when pre-dilation of stenoses is required to facilitate advance of the aspiration catheter.
As a rule, the venous outflow is always declotted before the arterial inflow. The first step involves placing the “safety” guidewire outside the introducer-sheath lumen and directly through the skin. This is done by introducing an angled tip hydrophilic guidewire through the one-way-valved “venous” introducer-sheath which already houses the raw metallic “safety” guidewire, the tip of which should be in the inferior or superior vena cava. The hydrophilic guidewire is steered into the central veins. The introducer-sheath is then completely withdrawn taking precaution not to accidentally pull out the two guidewires. It is then reinserted over the hydrophilic guidewire only (Fig. 12.1e). The “safety” guidewire now effectively enters the access through the skin puncture, alongside the introducer-sheath. It serves as a fluoroscopic landmark for the course of the venous lumen during aspiration and also guarantees that the access is rapidly and easily reopened by deploying a dilation balloon or stent in the case of a venous dissection, false guidewire tracking, or spasm that otherwise would cause irreversible access shutdown.
The technique of thromboaspiration revolves around a simple concept: a large caliber straight or slightly angled tip aspiration catheter, usually 6- to 9-F, with a rigid body but soft atraumatic tip is advanced through the venous introducer-sheath over the hydrophilic guidewire until it comes in contact with the apex or the most central segment of the thrombi (Fig. 12.1f). Use of a guidewire is not mandatory particularly when the access or arterialized vein has a straight course. Suction pressure is generated within the aspiration catheter by connecting it to a 50-mL Vaclock™ syringe (Fig. 12.1g) with a locking plunger kept in full deflation to create the necessary negative vacuum pressure effect (Appendix C: list of materials).
The catheter is pulled and pushed in rapid back-and-forth and rotary movements to macerate the thrombi and strip them off the vessel wall. The macerated debris as well as fresh blood are suctioned by the negative pressure created by the vacuum within the Vaclock syringe. The catheter is withdrawn either on return of fresh blood or after 5–10 s of aspiration. Both the contents in the syringe and the aspiration catheter are flushed through a gauze swab placed over a sterile plastic container on the work table to evaluate the amount of thrombi removed (Fig. 12.1h). The expulsion of larger clot fragments stuck in the lumen of the aspiration catheter may require applying considerable force on the syringe plunger and can be projectile. It is therefore a must that interventionists wear protective eye wear to prevent contamination of the eye from blood splash.
Angled tip aspiration catheters are most effective for thromboaspiration as their tip easily penetrates thrombi to reach the intimal lining and scrape off adherent mural thrombi in both veins and aneurysms. Aneurysmal thrombi removal is facilitated by applying manual compression over the aneurysm during the back-and-forth movements of the aspiration catheter to bring the catheter tip in closer contact with mural thrombi (Fig. 12.4b).
The sequence of inserting the aspiration catheter within the access lumen in contact with thrombi, maneuvering it back-and-forth and rotationally, withdrawing it, and expelling thrombi is repeated at lower segments until the whole access downstream to the introducer-sheath tip is declotted. Iodinated contrast is then injected under low pressure to fluoroscopically or angiographically confirm thrombi clearance and identify any underlying outflow stenosis. Any stenosis detected at this stage should not be dilated as it acts as a filter against any anastomotic thrombi that may inadvertently dislodge and embolize downstream to the lungs.
12.6.5 Arterial Inflow Thromboaspiration
The extraction of thrombi near the arteriovenous anastomosis starts only after thrombi have been removed in the venous outflow. The procedure is a repetition of the venous segment catheterization, whereby a hydrophilic guidewire is pushed through the hub of the “arterial” introducer-sheath already holding the raw metallic “safety” guidewire anchored in the proximal artery. The introducer is withdrawn and repositioned over the hydrophilic guidewire only (Fig. 12.1j). The aspiration catheter is then pushed, like in the venous phase, through the “arterial” introducer-sheath and over the hydrophilic guidewire toward the anastomosis. Thrombi located nearest to the introducer-sheath tip are aspirated first followed by those located within the arteriovenous anastomosis (Figs. 12.1k and 12.2e). Anastomotic thrombi (including the “arterial plug”) are always removed last. Early removal of anastomotic thrombi and subsequent restoration of the inflow has two consequences: it encourages residual venous thrombi to embolize more readily to the lungs, and it causes aspiration of a far larger volume of fresh blood than residual thrombi. Excessive aspiration of fresh blood is minimized by external manual compression/occlusion of the anastomosis.
12.6.6 Arterial Plug
Thrombi located at the arteriovenous anastomosis form a firm and rubbery plug rich in fibrin, red blood cells, and platelets. In the American literature, this is known as arterial plug or “bullet” [26]. These thrombi are very compact and adherent to the arterial wall and are therefore resistant to thrombolysis and aspiration (Figs. 12.2e, 12.3a–d, and 12.4a–d). Often they are macerated and partially detached but never completely removed (Figs. 12.3d, 12.4c). It is possible that some of these residual materials ultimately embolize to the lungs, the effect of which remains usually asymptomatic. Or they can embolize distally into the forearm arteries where their ill effects are more sinister especially when all three forearm arteries are involved (see further in this chapter). Historically, vascular surgeons have used 4–5-F Fogarty or over-the-guidewire Fogarty catheter to treat arterial plug. Thromboaspiration usually works equally well. Balloon dilation is also a valuable tool (Fig. 12.2f). Tretorola has described a Fogarty adherent clot catheter designed especially for this purpose [27]. On very rare occasions, a short stent needs to be placed to trap this arterial plug after failed attempts at mechanical removal. This short stent must never protrude into the artery.
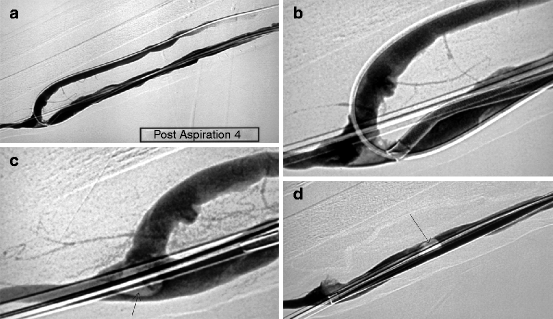
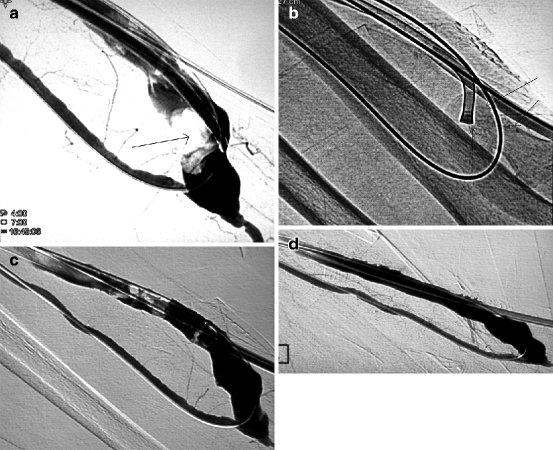

Fig. 12.3
(a) This straight PTFE graft was placed in the forearm as part of a distalization of anastomosis procedure on a hyper flow brachial–basilic fistula to treat distal ischemia. The graft became thrombosed and was thromboaspirated. However, an arterial plug remained at the level of its anastomosis with the radial artery. (b) An aspiration catheter was pushed in contact with the arterial plug. (c) Mechanical thrombectomy dislodged and pushed the thrombus (arrow) more distally temporarily. (d) Retrograde radial arterial flow finally pushed the thrombus into the graft (arrow) where it can be more easily removed

Fig. 12.4
(a) This angiogram shows a very resistant thrombus (arrow) lodged in an aneurysmal juxta-anastomotic segment of a left radial–cephalic fistula. (b) The trick is to apply manual compression over the aneurysm with one finger (arrows) to increase contact between the aspiration catheter and thrombi to facilitate their removal. (c) The thrombi are completely dislodged and partially aspirated. They are washed further downstream by the action of blood flow where they can be more easily aspirated as they are no longer stuck to the vessel walls. (d) The completion angiogram shows that almost all the thrombi have been removed
A dislodged thrombus may embolize onto the venous stenosis further downstream and reduce or obstruct access outflow once arterial inflow is restored. This embolized thrombus should be aspirated through the “venous” introducer-sheath in order to reestablish flow (Fig. 12.1n–p).
12.6.7 Dilation
Dilation of any underlining stenosis is performed once almost all thrombi in both the outflow and inflow segments have been removed. Appropriately sized dilation balloons are used to prevent any significant residual stenosis (Fig. 12.1q) with the exception of peri-anastomotic stenoses which must be deliberately underdilated (Fig. 12.2f). Like in any other form of dilation, the interventionist should have in stock dilation balloons of different sizes as well as high and ultrahigh pressure prototypes (e.g., Conquest® or Mustang®) in order to effectively and satisfactorily dilate the majority of stenoses. Stents are equally indispensable in the management of stenotic elastic recoil and severe venous rupture.
After aspiration and dilation of a thrombosed access, completion angiograms spanning the arteriovenous anastomosis and the superior vena cava should be performed and carefully studied (Fig. 12.8g–j). Residual clots or residual stenosis less than 50 % are usually acceptable in AVFs if overall flow is deemed satisfactory. However, there is a need for near perfection in prosthetic grafts, and, hence, further aspiration and use of larger and high-pressure dilation balloons (Fig. 12.1s–u) or even stents are necessary particularly if flow is sluggish. Grafts are more likely to rethrombose early and therefore require an almost perfect completion angiogram.
12.6.8 Arterial Embolism
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree