Reference
n
Inclusion criteria
Randomization
Definition of biochemical recurrence
Median follow-up
Biochemical progression-free survival (bNED)
Overall survival
Thompson et al. (2009), SWOG 8794
431
pT3 pN0 ± involved SM
60–64 Gy versus “wait and see”
>0.4
152 mo.
10 years: 53 versus 30 % (p < 0.05)
10 years: 74 versus 66 %
Median time: 15.2 versus 13.3 years
p = 0.023
Bolla et al. (2012), EORTC 22911
1005
pT3 ± involved SM pN0
pT2 involved SM
60 Gy versus “wait and see”
>0.2
127 mo.
10 years: 61 versus 38 % (p < 0.001)
81 versus 77 %
n.s.
Wiegel et al. (2013b), ARO 96-02
388
pT3 (± involved SM) pN0
PSA post RP undetectable
60 Gy versus “wait and see”
>0.05 + confirmation
112 mo.
10 years: 56 versus 35 % (p < 0.0001)
82 versus 86 %
n.s.
3.1 Southwest Oncology Group (SWOG) 8794
The SWOG 8794 was a randomized multi-institutional prospective trial of ART with 60–64 Gy versus observation alone for locally advanced prostate cancer following RP. Between 1988 and 1997, the study enrolled 425 patients with pathological stage T2 or T3 tumors who met at least one of the following pathological criteria: extracapsular extension, positive surgical margin, or seminal vesicle invasion. Pelvic lymph node dissection was obligatory, an undetectable PSA level before study entry was not mandatory. Thirty-three percentage of men in both arms had a serum PSA level >0.2 ng/ml at the time of randomization. A total 8 % of patients received pre-RP androgen deprivation therapy (ADT) (Thompson et al. 2006, 2009; van der Kwast et al. 2007).
Patients randomized to the ART arm began radiotherapy within 18 weeks after surgery. Treatment delivery was done utilizing 2D-based planning aimed at the prostatic fossa and paraprostatic tissues.
The primary end point in this study was metastasis-free survival. bNED was a secondary end point. A biochemical failure was defined as PSA level >0.4 ng/ml.
At the time of initial publication of the study (median follow-up 10.6 years), there was a significant benefit for patients treated with ART in terms of PSA relapse-free survival (median time to PSA relapse 10.3 vs. 3.3 years; p < 0.001) and recurrence-free survival (median time 13.8 vs. 9.9 years; p = 0.001).
In the observation arm, the use of salvage radiotherapy was not mandated by protocol. Ultimately a total of 70 men (33 %) received postoperative radiotherapy, mostly for a rising serum PSA level. The median PSA level at the time of salvage radiotherapy in these patients was 1.0 ng/ml, which would be considered ‘late’ salvage therapy by current standards.
By 5 years, twice as many men in the observation arm had received hormonal therapy versus in the ART arm (21 vs. 10 %; p < 0.001).
The initial report did not reveal advantage for ART concerning metastasis-free survival or overall survival. However, after a median follow-up of 12.5 years, a subsequent publication demonstrated a significant improvement in metastasis-free survival (12.9 years for the observation arm vs. 14.7 years for the ART arm; p = 0.016) as well as in overall survival in favor of ART (59 % for the ART arm vs. 48 %, observation arm; p = 0.023). The authors calculated that, on average, 12.2 patients had to be treated with ART to prevent one case of metastatic disease and 9.1 patients to prevent one death. It is interesting to note that the differences between the treatment groups become measurable not before 10 years, highlighting the importance of long-term follow-up in these patients.
However, the rate of observed distant metastasis was low (37 men in the observation arm and 20 men in the radiotherapy arm) and the majority of events in the analysis of metastasis-free survival and overall survival in both groups were deaths without evidence of metastatic prostate cancer (77 of 114 men in the observation arm and 73 of 93 men in the radiotherapy arm). Consequently, it has been argued that the survival benefit after ART was largely due to a lower rate of competing-cause deaths without evidence of distant metastasis, and that the impact of ART on metastatic disease and cancer-specific death was still uncertain.
3.2 European Organization for Research and Treatment of Cancer (EORTC) 22911
The EORTC 22911 was a phase III clinical trial of ART versus no immediate further treatment for patients with pN0 M0 prostate cancer with non-organ-confined disease (extracapsular extension or seminal vesicle invasion) or positive margins. All patients had ilio-obturator lymphadenectomy. A total of 1,005 men <75 years were accrued to the trial between 1992 and 2001. An undetectable PSA following prostatectomy was not mandatory for study enrollment. In total, 69.5 % of the patients had an undetectable PSA following RP. A total of 10 % of the patients received pre-RP ADT (Bolla et al. 2005, 2012).
For patients randomized to the ART arm, radiotherapy was initiated within 16 weeks following surgery, after recovery of urinary function. RT was delivered using 2D-based treatment planning to a total dose of 60 Gy over a period of 6 weeks.
The ‘revised primary end point’ of the study was PSA progression (initially it was metastasis-free survival), defined as an increase of more than 0.2 ng/ml over the lowest post-RP value measured on three occasions at least 2 weeks apart.
Overall, 301 (30 %) of men had a serum PSA level >0.2 ng/ml at the time of randomization (157 in the observation arm, 144 in the radiotherapy arm). In the observation arm, patients with biochemical or local recurrence were recommended to receive salvage radiotherapy. However, only 113 (51 %) of men with recurrent cancer after RP in the control arm received salvage radiotherapy, and 45 % of these received ‘late’ radiotherapy on the basis of clinically evident locoregional recurrence.
After a median follow-up of 5 years, biochemical progression-free survival (bPFS), clinical progression-free survival, and the cumulative rate of locoregional failures were significantly improved in the ART group (74 vs. 56 %; p < 0.0001 for bPFS). In total, 22.5 % of men in the observation arm subsequently underwent pelvic RT and 9 % eventually required hormonal treatment. Overall, the rates of distant metastasis (seen in 18 men in the observation arm and 19 in the radiotherapy arm) and deaths from prostate cancer (15 in the observation arm and 8 in the radiotherapy arm) as secondary endpoints in both arms were low and not significant different as well as in overall survival (p = 0.7).
Updated results with 10 -year follow-up data showed a continued bPFS advantage in favor of ART (61 vs. 41 %; p < 0.001) and a nonsignificant trend toward improved overall survival in the ART group (81 vs. 77 %; p > 0.1).
3.3 German Study Group (ARO 96-02/AUO AP 09/95)
The third phase III trial of ART versus a wait-and-see policy for patients with non-organ-confined prostate cancer (pathological stage pT3 pN0) with or without positive margins enrolled a total of 385 patients between 1994 and 2004. Approximately 11 % of patients received pre-RP ADT. All patients were required to have undergone a pelvic lymph node dissection. Unlike the SWOG and EORTC trials, patients were required to have an undetectable PSA following RP. Seventy-eight patients did not achieve an undetectable PSA and were excluded from treatment according to random assignment. Of the remaining 307 patients, 34 patients on the RT arm did not receive RT and five patients on the wait-and-see arm received RT. Therefore, 114 patients underwent RT and 154 patients were treated with a wait-and-see policy (Wiegel et al. 2009a, 2013b).
The primary end point of the study was bPFS. A biochemical failure was defined as a PSA increase out of the undetectable range with a consecutive confirmation.
Unlike the prior two studies, patients in this trial were treated with more modern 3D conformal RT. RT was prescribed to a dose of 60 Gy and initiated within 6–12 weeks following RP.
Over a median follow-up duration of 54 months, 67 progression events were observed in the observation arm and 38 in the radiotherapy arm, most of which were due to biochemical recurrence. Five-year progression-free survival was 54 and 72 % in the observation and radiotherapy arms, respectively (p = 0.0015). The benefit in favor of adjuvant radiotherapy was also observed when the 78 patients with persistent serum PSA elevation after radical prostatectomy were included in the analysis (p = 0.05), and persisted across all subgroups, with the exception of those with negative surgical margins. There was no benefit for metastasis-free survival or overall survival.
In the meantime, an update with data of 10-year follow-up was presented at the 2013 Genitourinary Cancers Symposium in Orlando, Florida. At 10 years, freedom from biochemical failure was achieved in 56 % of the adjuvant radiotherapy arm versus 35 % of the wait-and-see arm, for an absolute difference of 21 % favoring adjuvant treatment (p = 0.00002). No significant benefit was observed for adjuvant radiotherapy regarding metastasis-free survival or overall survival, though the trial was not powered to show this.
For patients with positive surgical margins, adjuvant radiotherapy had a clear advantage: Biochemical control was achieved in 55 versus 27 % of those in the wait-and-see arm, for an absolute difference of 28 %. Baseline factors associated with greater efficacy of adjuvant radiotherapy included higher Gleason scores, higher PSA levels, and more aggressive tumors. In a multivariate analysis, adjuvant radiotherapy reduced the risk of biochemical failure by 54 %. The relative risk of biochemical failure was reduced for patients with positive surgical margins, higher PSA level, stage T3a/b, and higher Gleason scores.
3.4 Clinical Trials Overview
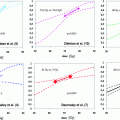
Patients with pT3 tumors and positive margins have been demonstrated to benefit most from ART (30 % bNED after 5 years) (Bolla et al. 2005; Thompson et al. 2006; Van der Kwast et al. 2007; Wiegel et al. 2009a). The 10-year follow-up data of all three trials confirm these results (Bolla et al. 2012; Wiegel et al. 2013b). In the prospective study of the South Western Oncology Group (SWOG), overall survival was improved from 13.5 years without to 15.2 years with ART (Thompson et al. 2009).
It is notable that the three randomized studies have used different definitions of biochemical progression: SWOG: PSA >0.4 ng/ml, EORTC: PSA >0.2 ng/ml, ARO/AUO: PSA >0.05 ng/ml.
Consequently biochemical recurrences (as an increase of PSA over the detection threshold) were detected earlier in the latter two studies, which explains the apparently worse results of the ARO study including patients with more favorable risk profile (undetectable PSA after RP) (Table 1).
In the EORTC and SWOG trials radiation was based on 2D treatment planning, where the prostatic fossa and paraprostatic tissue were targeted by using large treatment portals. Obviously, precise definition of target volumes was not essential, which is in great contrast to modern 3D conformal radiation treatment techniques such as IMRT. Compared to 2D-based planning, IMRT provides significant normal tissue sparing, but also demands exact definition of target volume.
Due to more precise techniques in treatment delivery, the Radiation Therapy Oncology Group (RTOG) (Michalski et al. 2010), the EORTC Radiation Oncology Group (Poortmans et al. 2007), and other cooperative groups (Wiltshire et al. 2007) have created consensus guidelines for delineation of target volumes for post-prostatectomy patients.
In 2011, Daly et al. reported the results of a meta-analysis of the three randomized clinical trials comparing radical prostatectomy alone to radical prostatectomy plus adjuvant radiation therapy for the treatment of men with prostate cancer and at least one of the following adverse pathologic features: extracapsular tumor extension, positive surgical margins, or seminal vesicle invasion. In total, 1,815 men were studied (385 from ARO, 1005 from EORTC, and 425 from SWOG). Analysis of oncological outcome was performed at 5- and 10-year time points. At this date, 10-year follow-up data were only available from the SWOG trial. An improved bPFS after ART could be demonstrated at 5 and 10 years with risk differences (RDs, risk difference is the risk in the treated group minus the risk in the control group) of 0.16 (95 % confidence interval [CI], 0.21–0.11) and 0.29 (95 % CI, 0.39–0.19), respectively. Furthermore, at 10 years, adjuvant radiation improved overall survival (RD: 0.11; 95 % CI, 0.20–0.02) and reduced the risk of metastatic disease (RD: 0.11; 95 % CI, 0.20–0.01) (Daly et al. 2011).
3.5 The Role of Positive Margins
Notably, central pathological review on the outcome at 5 years in the EORTC trial demonstrated positive surgical margins interacting statistically significantly with the treatment effect, to such an extent that the treatment benefit in patients with negative margins did not remain significant. The hazard ratio for the treatment benefit in the group with negative surgical margins was 0.87 (p = 0.601), compared to 0.38 (p < 0.0001) in the group with positive surgical margins according to the review pathology. Excluding the patients with a PSA of >0.2 ng/ml after prostatectomy, the hazard ratio for postoperative irradiation was 1.11 (p = 0.740) and 0.29 (p < 0.0001) for the patients with negative and positive margins, respectively (Van der Kwast et al. 2007). This benefit was also seen in the real adjuvant situation with the undetectable PSA before the start of radiation therapy (Wiegel et al. 2009a, b). After a median follow-up of nearly 5 years, there was a significant benefit from adjuvant radiation therapy for bNED: 72 versus 54 % (p < 0.03). In the subgroup of pT3 R1-tumors this benefit increased from 18 to 28 % (Wiegel et al. 2009a).
The location, the extent and the number of positive surgical margins after radical prostatectomy are significant predictors of biochemical progression after radical prostatectomy. The investigators of the Cleveland Clinic/Ohio found in their retrospective multi-institutional series of 7,160 patients treated with radical prostatectomy 1,540 patients with positive margins. The 7-year progression-free probability was 60 % in those patients, resulting in a hazard ratio for biochemical recurrence of 2.3 in the case of positive surgical margins compared with negative margins. The risk of biochemical recurrence was increased in patients with multiple versus solitary positive surgical margins (HR 1.4) and extensive versus focal positive surgical margins (adjusted HR 1.3) (Stephenson et al. 2009). Summing up the data from randomized trials and large retrospective series patients with positive margins and pT3-tumors have the largest profit from postoperative radiation therapy.
3.6 pT2 R1 Tumors
In the EORTC trial, when the data of patients with pT2 tumors and positive surgical margins were analyzed, there was a significant benefit with regard to 5-year biochemical progression-free survival rate in the irradiated group (76.4 vs. 52.2 % in the wait-and-see group) (Bolla et al. 2005). However, these data come from a subgroup analysis and biochemical progression-free survival was not the primary end point of this study. Therefore, the results must be interpreted with caution. The possible benefit of radiotherapy must be weighed out carefully in consideration of potential late effects as impaired erectile dysfunction.
4 The Impact of Pathology Review
The precise histologic assessment of RP specimens in patients with prostate cancer is of major importance for an accurate risk assessment of disease recurrence. The three histopathologic parameters of greatest prognostic importance are pathologic stage, Gleason score, and surgical margin status, where pathologic stage includes assessment for seminal vesicle invasion and extraprostatic extension. Several studies have previously evaluated interobserver variability between local pathologists and review pathologists in Gleason score in both the settings of needle biopsy and RP specimens (Allsbrook et al. 2001a, b; Glaessgen et al. 2004a, b; Oyama et al. 2005). In contrast, only five studies have evaluated interobserver variability in pathologic staging and margin status after RP (Van der Kwast et al. 2006; Ekici et al. 2003; Evans et al. 2008; Kuroiwa et al. 2010; Netto et al. 2011).
It is well known that pathology review has a significant impact on the results of randomized studies of definitive treatment of prostate cancer (Lawton et al. 2001). The RTOG trial 8531 randomized patients with locally advanced prostate cancer to either androgen suppression therapy (AST) or no AST after the administration of RT. In a subgroup of patients with pathologically reviewed biopsy specimens with Gleason score 8–10, there was a significant difference in overall survival (Lawton et al. 2001). However, comparable information is scarce concerning the postoperative treatment of prostate cancer.
The first published results came from the EORTC 22911 trial: 552 radical prostatectomy specimens (approximately 50 % of the patients) were retrospectively reviewed by a single pathologist with experience in urogenital pathology who examined all slides of the sample series (Van der Kwast et al. 2006, 2007). While there was a close concordance between local and review pathology regarding seminal vesicle invasion (94 %), less agreement was reached for extraprostatic extension (58 %) and for surgical margin status (69 %). An agreement rate cannot be given for the Gleason score, because it was not determined by the local pathologists in the EORTC trial (van der Kwast et al. 2006).
Biochemical progression was significantly delayed in all subgroups of men treated with adjuvant radiotherapy in EORTC 22911 (p ≤ 0.02 for all comparisons) (Bolla et al. 2005). However, the subsequent retrospective study involving central pathology review found that only surgical margin status was significantly associated with a benefit of adjuvant radiotherapy treatment (p < 0.01), and that the treatment benefit in patients with negative margins was not significant, irrespective of other risk factors (p = 0.6) (Van der Kwast et al. 2007). Among patients with positive surgical margins, a beneficial effect on biochemical recurrence was seen with adjuvant radiotherapy treatment in men with high Gleason score cancers and those with seminal vesicle invasion.
In the German ARO/AUO study, a prospective pathology review was performed on 85 % of RP specimen of 307 patients with undetectable PSA to investigate the influence of pathology review on the analysis. There was a fair concordance between pathology review and local pathologists for seminal vesicle invasion (91 %), surgical margin status (84 %), and for extraprostatic extension (75 %). Agreement was much less for Gleason score (47 %), whereby the review pathology resulted in a shift to Gleason score seven. In contrast to the analysis of progression-free survival with local pathology, the multivariate analysis including review pathology reveals positive surgical margins and Gleason score >6 as significant prognostic factors (Bottke et al. 2013b). The authors conclude, that phase 3 studies of postoperative treatment of prostate cancer should be accomplished in the future with a pathology review. In daily practice, a second opinion by a pathologist experienced in urogenital pathology would be desirable, in particular, for high-risk patients after RP.
This is why the PREFERE study has included pathology review as a mandatory step for study inclusion in the design of the nationwide German prostate cancer trial Evaluation of Four Treatment Modalities in Prostate Cancer with Low or “Early Intermediate” Risk (PREFERE), which has just opened (Wiegel et al. 2013a; Bottke et al. 2013a). PREFERE is a prospective randomized multicenter trial developed to compare the four possible treatment options currently recommended by the European guidelines (Heidenreich et al. 2011) for favorable risk prostate cancer (radical prostatectomy, external beam radiotherapy, permanent seed implantation, and active surveillance) (Wiegel et al. 2013a).
5 Optimal Radiation Dose
To date there is no established consensus regarding the optimal prescription dose for adjuvant radiotherapy. Petrovich et al. have indicated that even low doses in the range of 45–50 Gy are beneficial in terms of local control and disease-free survival (Petrovich et al. 1991, 2002). The findings of the three randomized studies have been obtained with a prescription dose of 60 Gy with conventional irradiation over 6 weeks.
Based on American Society of Therapeutic Radiation Oncology recommendations, a dose of 64 Gy or higher (with conventional fractionation) should be prescribed (Thompson et al. 2013). Valicenti and Gomella have demonstrated evidence of improved biochemical outcomes using higher radiation doses. Despite higher doses, in fact, treatment is generally well tolerated with minimal late severe toxicity (Valicenti and Gomella 2000).
6 Adjuvant RT of Pelvic Lymph Nodes?
The three randomized trials included only patients with cN0 or pN0-disease. The effect of adjuvant RT in node-positive prostate cancer has not yet been prospectively assessed. However, there are interesting retrospective data raising the question whether men with nodal involvement confirmed during prostatectomy could benefit from adjuvant RT. A recent retrospective study reported a significant positive impact of RT in combination with hormonal therapy in patients with nodal metastases treated with RP and pelvic lymph node dissection (Da Pozzo et al. 2009). However, this study was limited by a potential patient selection bias mainly due to its retrospective and unmatched design. In fact, patients treated with adjuvant RT were those affected by more aggressive disease. For this reason, no effect of adjuvant RT on cancer-specific survival was demonstrated on univariate survival analyses. There was significant gain in predictive accuracy when adjuvant RT was included in multivariable models predicting biochemical recurrence-free and cancer-specific survival (gain: 3.3 and 3 %, respectively; all p < 0.001).
In a huge retrospective series, Briganti et al. assessed the effect of adjuvant RT in node-positive prostate cancer including two homogeneous matched patient cohorts exposed to either adjuvant RT plus HT or adjuvant HT alone after surgery. In this series from Milan and Jacksonville a total of 703 patients were treated, with a median follow-up of 95 months. Patients were matched for age at surgery, pathologic T stage and Gleason score, number of nodes removed, surgical margin status, and length of follow-up. The overall survival advantage was 19 % in favor of adjuvant radiation therapy plus hormonal treatment compared with hormonal treatment alone. Similarly, higher survival rates associated with the combination of HT plus RT were found when patients were stratified according to the extent of nodal invasion (namely, ≤2 vs. >2 positive nodes; all p ≤0.006) (Briganti et al. 2011). Because of the retrospective nature of this series with no standardized definition of target volumes, radiation dose and duration of hormonal treatment, these results should be interpreted with caution. However, it provides support for this treatment in selected cases, whereas it should be validated in prospective clinical trials.
7 Additional Use of Hormone Therapy to ART
It is now clearly established that the standard nonoperative management for patients with locally advanced prostate adenocarcinoma includes long-term ADT. Two previous cooperative group trials have demonstrated an overall survival advantage for high-risk patients with an intact prostate treated with 2–3 years of ADT as compared to patients treated with short-term ADT (Bolla et al. 2009; Horwitz et al. 2008). It remains unknown if there is a benefit for the addition of adjuvant ADT for men with high-risk, node negative prostate adenocarcinoma initially treated with RP and pelvic lymph node dissection. The primary rationale for use of ADT post-RP is to (1) improve local control by eradicating disease in a hypoxic scar that may be radioresistant; (2) address micrometastatic disease which may have spread to the lymph nodes or distant sites; and (3) alter PSA kinetics in patients who will eventually relapse (Hanlon et al. 2004; Kaminski et al. 2003; Rossi et al. 2011).
Previous studies have indicated a potential benefit for men at high risk of recurrence treated with combination therapy. A secondary analysis of patients status-post an RP enrolled on Radiation Therapy Oncology Group (RTOG) 85-31 (Corn et al. 1999), a phase III trial comparing standard external beam RT plus immediate ADT versus RT alone for patients with nonbulky prostate cancer, found a biochemical control advantage for patients who received combination therapy as compared to men treated with RT alone. With a median follow-up of 5 years, the progression-free survival for men treated with combination therapy was estimated to be 65 % as compared to 42 % for men treated with RT alone (p = 0.002). Similar results were seen in a retrospective study performed at Stanford University (King et al. 2004). A subsequent RTOG study (P-0011) was designed to determine the benefit of combination therapy for man with unfavorable prognostic factors and an undetectable PSA treated with ART. This trial was unfortunately closed due to poor accrual (Elshaikh et al. 2011).
Recently, Abdollah et al. evaluated the long-term survival of prostate cancer patients who have experienced biochemical recurrence after RP and ART. Patients with a short time to biochemical recurrence, a Gleason score of ≥8 and ≥2 positive lymph nodes had lower survival rates than other patients (Abdollah et al. 2013).
In ongoing EORTC trial 22043, patients with Gleason score 5–10, undetectable PSA and pathological stage pT2R1 or pT3a-b will be randomized within 3 months after radical prostatectomy between postoperative irradiation alone or postoperative irradiation and short-term adjuvant androgen deprivation for 6 months. The primary trial endpoint is 5-year biochemical progression-free survival.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree