Abstract
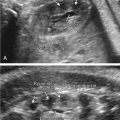
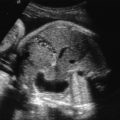
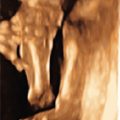
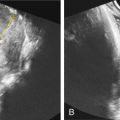
Tuberous sclerosis is a disorder of cellular differentiation, proliferation, and migration in early development characterized by the formation of benign, harmartomatous lesions in virtually any organ system. Tuberous sclerosis is inherited in an autosomal dominant fashion, although sporadic mutations are found in over two-thirds of patients. Diagnosis can be made through (1) identification of a mutation in one of the two identified responsible genes, TSC1 and TSC2 , or (2) clinical findings of defined major and minor criteria. Organ systems most commonly affected include the central nervous system, skin, heart, kidneys, bones, and blood vessels. Despite complete penetrance, the phenotypic variability is quite high, leading to a wide spectrum of clinical manifestations as well as heterogeneity in symptom onset and severity. Cardiac rhabdomyomas are a common sonographic prenatal finding in cases of tuberous sclerosis. Fetal MRI can also be used as an adjunct to prenatal sonography to evaluate for intracerebral signs of tuberous sclerosis. Prenatal diagnostic testing is available and should be considered in patients with a personal or family history of the disease or in cases of suspicious prenatal findings.
Keywords
tuberous sclerosis, rhabdomyoma, angiomyolipoma, TSC
Introduction
Tuberous sclerosis, also known as the tuberous sclerosis complex (TSC), is a disorder of cellular differentiation, proliferation, and migration in early development that variably affects multiple organ systems. First described in 1862 by Friedrich von Recklinghausen, TSC is characterized by the formation of benign, hamartomatous lesions in virtually any organ system, most commonly the central nervous system (CNS), skin, heart, lungs, kidneys, bones, and blood vessels. The inheritance pattern of TSC is autosomal dominant, although sporadic mutations are found in over two-thirds of affected patients. Despite complete penetrance, the phenotypic variability is quite high, leading to a wide spectrum of clinical manifestations as well as heterogeneity in symptom onset and severity.
Disorder
Definition
Diagnostic criteria for tuberous sclerosis require either: (1) genetic testing identifying a known pathogenic mutation/disruption in either TSC1 or TSC2 or (2) a clinical diagnosis based on established major and minor criteria ( Table 143.1 ). A “definite” diagnosis of tuberous sclerosis is considered in the presence of two major features or one major feature with two or more minor features. A “possible” diagnosis is defined as having either one major feature or two or more minor features.
| Major Features | Minor Features |
|---|---|
| Hypomelanotic macules (≥3, at least 5-mm diameter) | “Confetti” skin lesions |
| Angiofibromas (≥3) or fibrous cephalic plaque | Dental enamel pits (>3) |
| Ungual fibromas (≥2) | Intraoral fibromas (≥2) |
| Shagreen patch | Retinal achromic patch |
| Multiple retinal hamartomas | Multiple renal cysts |
| Cortical dysplasias (including tubers and white matter radial migration lines) | Nonrenal hamartomas |
| Subependymal nodules | |
| Subependymal giant cell astrocytoma | |
| Cardiac rhabdomyoma | |
| Lymphangioleiomyomatosis (LAM) * | |
| Angiomyolipomas (≥2) * |
* Combination of these two major features without other features does not meet criteria for a definite diagnosis
Prevalence and Epidemiology
TSC is the second most common neurocutaneous syndrome after neurofibromatosis type 1. The prevalence is approximately 1 : 6000 live births, with over 1.5 million people affected worldwide. There is no gender or race-specific bias.
Etiology and Pathophysiology
Two responsible genes have been identified in the pathogenesis of tuberous sclerosis, TSC1 (9q34) and TSC2 (16p13.3). These genes encode the proteins hamartin and tuberin, which together form an intracellular complex which functions as a tumor suppressor of the mTOR (mammalian target of rapamycin) kinase cascade. The mTOR cascade plays a crucial role in regulating cell growth and proliferation and is thought to play an important role in neural stem cell production and migration. Dysfunctional gene products from mutations in either TSC1 or TSC2 lead to upregulation of the mTOR kinase cascade and subsequent disorganized cellular growth and differentiation. A mutation in each allele at either the TSC1 or TSC2 loci is necessary for the expression of TSC (i.e., “two-hit” hypothesis) with the first being a germline mutation followed by a somatic mutation of the remaining allele.
There is no definitive correlation between genotype and phenotype, although mutations or deletions in TSC2 tend to have more severe clinical manifestations. Mutations in TSC2 are more common and more often tend to be sporadic. Deletions also tend to be the more common type of mutation in TSC2 and may involve neighboring gene PKD1 (responsible for polycystic kidney disease). Individuals with this contiguous gene deletion will often present with renal cysts in early childhood and have a more severe renal phenotype than the renal disease observed in patients with TSC. In contrast, mutations in TSC1 tend to be familial. No identifiable mutation is found in 10%–15% of TSC patients; therefore diagnosis is made based on clinical features alone. These patients tend to have milder clinical manifestations of disease. It is thought that mosaicism or mutations in the regulatory and intronic components of TSC1 and TSC2, as opposed to a potential third candidate gene, account for the percentage of cases that have no identifiable mutation.
Manifestations of Disease
Clinical Presentation
The classically described triad of epilepsy, mental retardation, and facial angiofibromas is only observed in 30%–40% of patients with TSC. Greater than 85% of affected patients will have some CNS manifestation of disease and more than 90% will have cutaneous findings. CNS lesions and renal complications are the leading causes of morbidity and mortality in TSC. The majority of patients are diagnosed during childhood, but given the extreme phenotypic variability of disease, a proportion of patients are diagnosed only when genetic testing is used secondary to diagnosis in another family member. Significant clinical manifestations and findings are listed in Table 143.2 , categorized by organ system.
| Central nervous system | Seizures, developmental delay, intellectual disability, autism, neuropsychiatric disorders, retinal achromic patch, cortical and subcortical tubers/dysplasias, subependymal nodules, subependymal giant cell astrocytomas, retinal hamartomas |
| Dermatologic system | Hypomelanotic macules (ash leaf spots), angiofibromas, adenoma sebaceum, fibrous cephalic plaques, raised connective tissue nevi (shagreen patches), ungual fibromas, confetti skin lesions, café au lait spots, dental pits |
| Renal system | Renal cysts, angiomyolipomas (with associated risk of life-threatening hemorrhage and renal failure or hypertension owing to mass effect), increased lifetime risk of renal cell carcinoma |
| Cardiopulmonary system | Lymphangioleiomyomatosis (proliferation of atypical smooth muscle cells in the lymphatics of the lungs leading to interstitial lung disease and cystic lung destruction), cardiac rhabdomyomas, cardiac angiolipomas |
| Skeletal system | Localized areas of sclerosis |
| Vascular system | Vascular dysplasia, aneurysms (intracranial, thoracic, abdominal) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree