Abstract
Twin reversed arterial perfusion (TRAP) sequence is a rare complication specific to monochorionic twin pregnancies in which a dysmorphic acardiac fetus receives circulatory support from a pump twin via aberrant placental arterioarterial anatomoses. The hemodynamic burden of supporting an acardiac mass places the pump twin at risk for polyhydramnios, heart failure, hydrops, and death. A TRAP diagnosis is secured by ultrasound, with necessary findings including a normal-appearing pump fetus and a grossly abnormal acardiac twin in the setting of a monochorionic pregnancy, with color Doppler study demonstrating reversal of arterial flow to the acardiac fetus. Due to an increased rate of aneuploidy, invasive genetic testing is recommended for pregnancies complicated by TRAP sequence. Therapy with invasive cord occlusion to sever the intertwin vascular circuit should be considered with a relatively large acardiac twin or when pump twin compromise is suspected at a previable gestational age, with radiofrequency ablation (RFA) a commonly used contemporary cord occlusion strategy for this presentation.
Keywords
twin reversed arterial perfusion sequence, TRAP sequence, acardiac twin, monochorionic twin pregnancy
Introduction
Twin reversed arterial perfusion (TRAP) sequence is a rare complication specific to monochorionic twin pregnancies. As a result of early vascular disruption occurring during embryogenesis, a dysmorphic acardiac twin receives circulatory support from a pump twin via aberrant placental arterioarterial anastomoses. Because the acardiac twin is entirely dependent on perfusion from the pump twin, the pump twin is at risk of hemodynamic compromise.
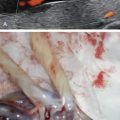
Originally described as acardia, the condition was classified further according to the overall appearance of the acardiac fetus on postmortem and radiographic evaluation. The most common presentation, acardius acephalus, consists of a well-formed fetal pelvis and lower limbs without a head or skull and usually without thoracic organs and arms ( Fig. 163.1 ). However, this classification system has been supplanted by the broader, albeit less descriptive, TRAP acronym.
Disease
Prevalence and Epidemiology
The estimated occurrence of TRAP sequence is approximately 1 : 35,000 pregnancies, including 1 : 100 monozygotic twins and 1 : 30 monozygotic triplets. Most (74%) TRAP presentations occur within monochorionic diamniotic pregnancies, with the remaining cases monoamniotic. Although it is generally accepted that acardia is unique to monochorionic pregnancies, there are two reported cases within dichorionic twin pregnancies.
Etiology and Pathophysiology
Theories exist to explain disease pathogenesis; however, the precise etiology of TRAP is unknown. The most widely accepted theory is that the condition is the result of abnormal arterioarterial anastomoses established early in embryogenesis between twin fetuses sharing a single placenta. The acardiac mass lacks direct placental perfusion and is dependent on a retrograde arterial supply of relatively deoxygenated blood from the pump twin. Asymmetric circulation favoring caudal aspects of the acardiac fetus and tissue hypoxia are believed to contribute to resultant fetal dysmorphology. Excess circulatory workload can prove detrimental to the recipient twin, which is at increased risk of high-output cardiac failure. An alternative, although less widely supported, hypothesis for disease pathogenesis involves a primary defect in cardiac embryogenesis, which secondarily permits retrograde blood flow within the acardiac twin.
Manifestations of Disease
Clinical Presentation
TRAP presentation includes a normal-appearing (pump) fetus and a grossly abnormal (acardiac) fetus in the setting of a monochorionic pregnancy. Although the acardiac fetus may possess some discernible features, it either lacks a heart or possesses a rudimentary cardiac structure. In some cases, the acardiac twin may appear only as an amorphous tissue mass. There is a predisposition toward acardiac twin deformities of the head, trunk, and upper extremities. Other common findings within the acardiac mass include subcutaneous edema, cystic inclusions within the trunk, and general hydropic changes ( and ![]()
![]() ).
).
Imaging Technique and Findings
Ultrasound.
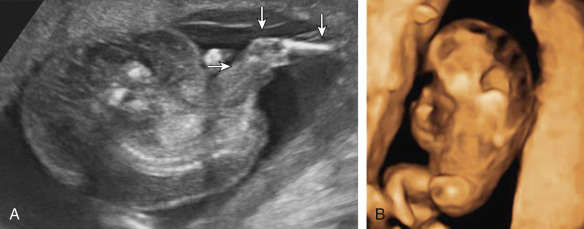
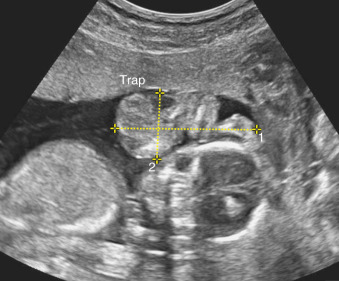
Although ultrasound (US) diagnosis of a TRAP sequence usually involves showing absent cardiac activity within an acardiac fetal mass that possesses reversed umbilical artery blood flow on Doppler study, the identification of acardiac fetal cardiac activity does not altogether preclude this diagnosis. Cardiac activity in the recipient is infrequently observed, and may be the result of rudimentary heart function or transmitted arterial pulsations from the pump twin ( Fig. 163.2 ).
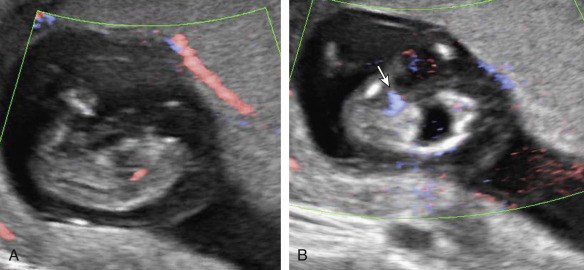
Because of excess circulatory demands on the pump twin, it is prone to the development of high-output cardiac failure. Given that there is no chance of ex utero survival for the acardiac fetus, this potential complication is of paramount importance when managing TRAP presentations. US evidence of cardiovascular deterioration or frank cardiac failure in the pump twin includes the detection of polyhydramnios, cardiomegaly or cardiac dysfunction, abnormal fetal fluid collections, hydrops, and abnormalities of the ductus venosus Doppler waveform.
In addition to changes reflective of circulatory failure, the pump twin is at increased risk for major anatomic malformations and aneuploidy. Review of case reports with known karyotype information of pump and acardiac fetuses revealed the following abnormalities: monosomy, trisomy, deletions, mosaicism, and polyploidy, with almost 9% of pump fetuses being karyotypically abnormal. In addition to comprehensive fetal anatomic surveys, invasive genetic testing for karyotype determination is recommended. Fetal echocardiography is also recommended in the evaluation of TRAP pregnancies, owing to the potential for pump twin cardiac dysfunction but also because of a known increase in risk of cardiovascular malformation in monochorionic twins.
Abnormalities of the umbilical cord may be present in both twins. A single umbilical artery is detected in approximately two-thirds of cases. Reports exist of TRAP pregnancies involving a conjoined cord, such that the umbilical cord of the acardiac twin directly anastomoses with the pump twin umbilical cord without any interface along the placental surface.
As a result of gross deformity, conventional biometric parameters are not applicable when assessing acardiac fetuses. Alternative formulas have been devised to estimate weight of the acardiac fetus; however, because of the protean morphologies of acardiac twins, the accuracy and generalizability of these formulas are unproven. An example of a commonly used formula is: weight (g) = 1.2 × length (cm) − 1.66 × length (cm), where the length used is the longest linear measurement ( Fig. 163.3 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree