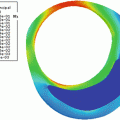
Fig. 15.1
Methods for stenosis quantification in angiography (a) and their equivalents in ultrasonography (b). NASCET, method used in the American studies NASCET and ACAS; ECST, method used in the European study ECST; (CCA), method that uses the distal CCA diameter as the reference; CCA, common carotid artery; CE, external carotid artery; CI, internal carotid artery
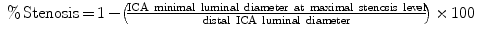
In ECST the minimal luminal diameter at the level of stenosis was compared with the diameter of the carotid bulb at the level of stenosis according to the formula:
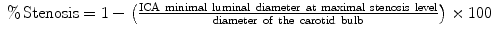
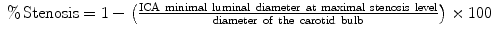
This methodological difference led to an underestimation of stenosis in the NASCET study when compared to the ECST. For example, a 70% NASCET1 stenosis corresponds to 85% ECST (see footnote 1) and a 70%E corresponds to 40%N stenosis (Table 15.1). For more severe lesions this difference tends to be less relevant and above 70%N all the lesions are also >70%E.
Table 15.1
Theoretical correspondence of the percentage of stenosis calculated according to the American (NASCET) and European (ECST) methods
Study | Percentage of stenosis | |||||||
|---|---|---|---|---|---|---|---|---|
NASCET | 11 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
ECST | 50 | 65 | 70 | 75 | 80 | 85 | 91 | 97 |
However, only stenoses >85%E are also >70%N, suggesting that the population of both trials was not exactly comparable. Lesions 70–85%E correspond to 50–70%N, and in fact a later analysis of the NASCET trial showed a significant benefit of CEA in this group of patients.
The understanding of all these discrepancies is very important, as the correct quantification of the degree of stenosis remains the key parameter used for therapeutical decision [6–9]. On the other hand, angiography is not used anymore in general clinical practice for the purpose of stenosis quantification, being replaced by other methods like color-flow duplex scan (CFDS), angio-magnetic resonance (Angio-MR), or angio-computerized tomography (Angio-CT) [10, 11].
2 Quantification of Carotid Stenosis Using Color-Flow Duplex Scan
The use of CFDS as a screening and also as a treatment decision method prompted the introduction of quantification criteria, which were considered accurate when compared with angiography, and associated with high sensitivity, with low rate of false positives. Also, the therapeutical decision-making strategy based on CFDS requires the need of diagnostic criteria with high specificity and negligible number of false negatives.
In view of the generalized use of CFDS examination, a reappraisal of the diagnostic parameters for stenosis measurement is pertinent.
CFDS is a unique method to study arterial stenosis as it permits the assessment of a number of morphologic and hemodynamic aspects related to the lesion.
It may visualize the plaque longitudinally, allowing the quantification of stenosis according to the angiographic criteria used in NASCET and ECST. In fact, the minimal luminal diameter can be compared to the clearly visualized bulb diameter (European criteria), to the distal common carotid artery (CCA), and also to the distal ICA diameter (American criteria) (Fig. 15.1b).
Beyond this, the carotid axis can also be evaluated in transversal views, providing cross-sectional assessments of the stenosis (Fig. 15.2). After identifying the point of tighter stenosis, one can measure the obstruction comparing the minimal luminal area with the outer arterial area (cross-sectional area measurement).
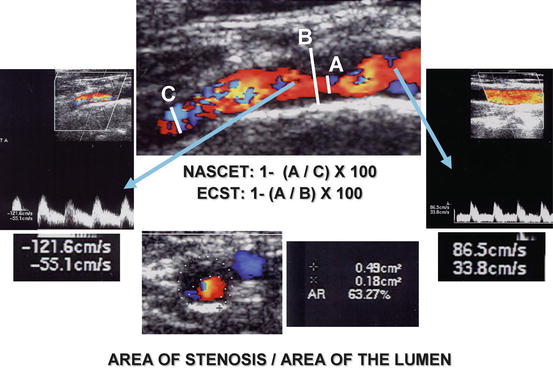

Fig. 15.2
Quantification of carotid stenosis by CFDS, according to the linear longitudinal methods (NASCET and ECST), according to the cross-sectional area measurement, and according to the hemodynamic assessment
Finally, the CFDS is also able to evaluate the flow repercussion caused by the obstruction by quantification of flow velocities, thus providing hemodynamic objective parameters, which correlated well with the degree of stenosis, allowing its classification in different subgroups of severity[12].
The quantification of carotid stenosis by CFDS should include and combine the information emerging from morphologic and hemodynamic assessments, thus incorporating the unique capabilities of the method on the decision process[13].
3 Hemodynamic Assessment
Several hemodynamic criteria were introduced to quantify carotid stenosis. They include the systolic and/or diastolic velocities as well as relations between velocities obtained at different levels (Fig. 15.2).
The more useful parameters were shown to be the peak systolic velocity (PSV) and the end-diastolic velocity (EDV) measured immediately after the more stenotic part of the lesion.
In the 1980s, D. E. Strandness introduced the first original criteria based on flow velocity to classify carotid stenosis according to the cutoffs: A: Normal; B: 1–15%; C: 15–50%; D: 50–80%; D+: 80–99% [14].
Other authors, aiming to differentiate the levels of stenosis that were found to be clinically relevant after the multicenter trials, proposed other criteria based on PSV, EDV, or combinations of these values [15].
Flow acceleration with increase of PSV appears with diameter reduction of >50% (>75% area reduction) and it seems to be one of the earliest hemodynamic manifestations of a significant lesion. EDV increase is a later feature as only severe stenosis (>70% diameter reduction) is related to the persistence of gradient during diastole, meaning a lower distal peripheral resistance.
The measurement of proximal velocities is usually performed at the CCA and is used to compare with velocities obtained at the level of the stenosis or 1 cm beyond. This is the location where higher velocities can be found.
Fujitani [16] called the attention to the normal increase of ipsilateral carotid velocities when the contralateral carotid artery is occluded. This fact requires reappraisal of the criteria for stenosis quantification in the presence of severe contralateral disease. Others [17, 18] suggested the importance of using indexes comparing velocities at the stenosis with the ones obtained in the CCA. Examples of these ratios are the Systolic Index (PSVica/PSVcca), the Diastolic Index (EDVica/EDVcca), and the PSVica/EDVcca Index.
Refinement of these criteria aimed to improve the diagnostic accuracy of the relevant cutoffs for symptomatic and asymptomatic carotid stenosis (50% [4], 60% [3], and 70% [1, 2]), which could have impact on clinical decision.
The pitfalls [9, 10, 19] of these investigations are related to the multiplicity of criteria and protocols suggested for the CFDS examination, and also by the lack of uniformity in the validation process against angiography, which depends largely on the quantification technique and criteria used (NASCET, ECST, or CCA criteria?). This controversy is very relevant, as in later years CFDS was established as the major technique used in clinical practice for the management of patients with carotid stenosis and correlation of flow velocities with diameter reduction according to ECST or NASCET criteria and area reduction—the four more significant parameters—became imperative.
4 Morphologic Assessment: The Advantages of Cross-sectional Area Measurement
The measurement of stenosis by bidimensional (2D) ultrasonography may include longitudinal and transversal evaluations.
The assessment of the carotid axis in longitudinal plane allows the definition and quantification of the minimal luminal diameter, which is then compared with the diameter of the bulb, the ICA, or the CCA. This evaluation is always dependent on a subjective choice of the operator according to his perception of the higher stenosis point. When the lumen is regular and circular the determination of the minimal luminal diameter is predictable and independent on the chosen plane of assessment. However, very often, lesions are complex and eccentric and the geometry of the lumen in the region of the plaque is elliptical or irregular, rendering the calculation of the minimal luminal diameter highly dependent on the plane chosen and subject to remarkable variations (Fig. 15.3). Porsche et al. [20] found that the lumen was not circular in 18% and the ACSEPT study [21] characterized the carotid lumen at the level of the stenosis and demonstrated that it was circular in 28%, eliptical in 50%, and irregular in 22%. This is in consonance with the need to evaluate the luminal shape and to assess its repercussion on the longitudinal plane used for diameter quantification.
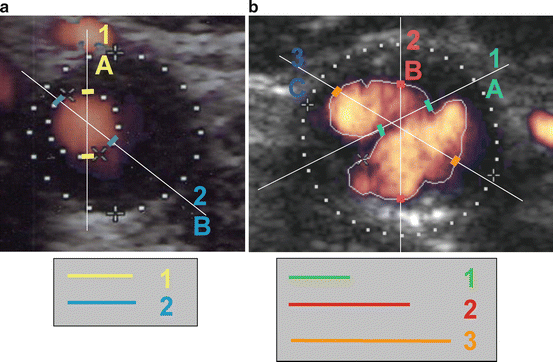

Fig. 15.3
(a) Cross-sectional image of a stenosis with regular and circular lumen: both linear diameters (1, 2) are identical. (b) Cross-sectional image of a stenosis with irregular lumen: the three diameters (1, 2, 3) are significantly different
Therefore, characterization of the lumen geometry is imperative and is better accomplished using a cross-sectional approach that permits the choice of the point where the stenosis is tighter. Severity of stenosis should then be assessed by the ratio of the area at the minimal lumen and the area obtained from the peripheral contour of the artery. In the future, a tridimensional (3D) assessment of plaque structure and lumen may provide more realistic estimation of the degree of stenosis as well as the total volume of the lesion [22].
Physiologically, the cross-sectional area reduction is the most relevant parameter to induce hemodynamic effect, and, therefore, area measurement appears to be more adequate to quantify carotid stenosis, because it is independent on the longitudinal plane used for the evaluation.
This method of quantification of stenosis using area measurements was recognized as being superior to the linear measurement, in studies based on the reconstruction of the plaques removed by CEA [13, 23, 24].
Another approach to challenge the longitudinal diameter method was made by Alexandrov et al. [13] who compared several parameters: angiographic longitudinal measurements by the NASCET and ECST methods, area quantification extrapolated by the formula πr 2, ultrasonographic velocity assessment, determination of stenosis by conversion to area using diameter–area conversion tables, area quantification after assembling and measuring removed surgical specimens, and finally the subjective (“eye-driven”) method for calculation on angiography.
The authors concluded that both NASCET and ECST methods underevaluate the lesions, when comparisons were made with CEA specimens. Area of stenosis as extrapolated from the angiography-driven NASCET and ECST diameters as well as from CFDS diameters was not significantly different from area measurements in CEA specimens. Using velocity parameters, the linear NASCET measurement had a correlation of 0.75, higher than the one obtained from linear ECST method (r = 0.57). Area extrapolation (πr 2) method correlated well with the NASCET (r = 0.92) and ECST (r = 0.63) methods.
The limitation of this study derives from the inaccuracy of the extrapolation of diameter measurements to area in irregular lesions [20, 21], but points out that assessments based on area reduction calculations are closer to the reality from endarterectomy specimens [23]. However, the obvious drawbacks result from the difficulty to perfectly assemble surgical specimens, with inevitable modifications and distortions in the size and shape of the structure of the lesion.
These studies suggest the need to define new gold standards to compare and validate the different methods for carotid stenosis quantification.
The aim of our research was to compare the different measurement methods of carotid stenosis with area ratios at the point of highest velocity acceleration obtained intra-operatively with the probe applied directly over the artery, which gives a real image of the lesion area (and “volume”) [25] in physiological conditions.
5 A New Gold Standard: Intra-operative Cross-sectional Area Measurement [25]
The intra-operative CFDS (IO CFDS) was performed during the procedure of CEA, after the dissection of the carotid bifurcation but before arteriotomy, thus obtaining in vivo high-definition images of the plaque and stenosis, as the sterile probe is directly applied over the artery (Fig. 15.4).
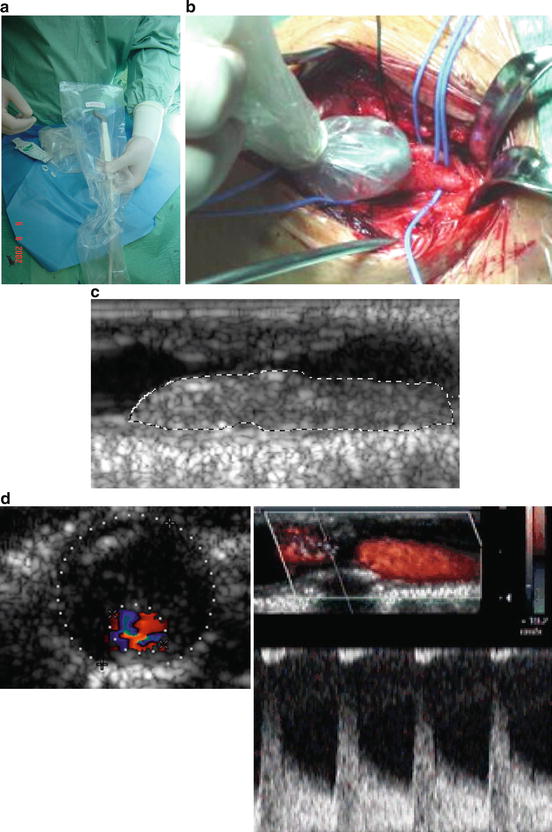

Fig. 15.4
Intra-operative CFDS. (a) Specially designed “hockey-stick” probe and the plastic kit for surgical use; (b) detail of the examination; (c) plaque structure characterisation; (d) quantification of stenosis morphologically (área) and hemodynamically
This approach eliminates the distortion associated with the models using the in vitro reconstruction of the removed plaque by endarterectomy. On the other hand, the IO CFDS permits the easy identification of the higher stenotic point and the direct quantification of the luminal and arterial area and thus the determination of the percentage of obstruction. This IO CFDS evaluation can afterwards be compared with the transcervical area measurement and we assumed that it represents the most reliable method to determine the degree of carotid stenosis.
The technique used for IO CFDS included an equipment ATL-Philips® HDI 3000, a 5–10 MHz multifrequency probe specially designed for intra-operative studies, and a kit for sterile surgical protection of the probe and cable (Fig. 15.4a, ba, b).
After arterial exposure the probe is located directly over the carotid axis and high-quality images of the lesion are obtained (Fig. 15.4cc). Then, the point of higher stenosis is located in cross-sectional views and another image is taken after ensuring that the probe is located perpendicularly to the artery. The quantification of the percentage of stenosis was determined in this image using the area measurement technique (Fig. 15.4dd).
6 Comparison of Conventional Methods for Stenosis Quantification with IO CFDS Cross-sectional Area Measurement [25]
A study was conducted to validate conventional morphologic and hemodynamic criteria for stenosis quantification against the new gold standard (cross-sectional area measurement in IO CFDS evaluation).
The study included the comparison of IO CFDS with three parameters: (1) transcervical cross-sectional area measurement; (2) transcervical longitudinal diameter measurement according to the NASCET and ECST methods; and (3) commonly used hemodynamic criteria (Tables 15.2, 15.3, and 15.4).
Table 15.2
Hemodynamic criteria A14 (University of Washington)
Degree of stenosis (%) | |||
|---|---|---|---|
NASCET | ECST | PSV (cm/s) | EDV (cm/s) |
• <11 | • <50 | <125 | <140 |
• 11–60 | • 50–80 | >125 | <140 |
• >60 | • >80 | >125 | >140
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|