Chapter NINE
Ultrasound
CLARE HAYSTEAD  CHAPTER EDITOR
CHAPTER EDITOR
CLARE M.
HAYSTEAD
HISTORY
A 28-year-old female presents with vaginal bleeding and a beta-human chorionic gonadotropin (0-HCG) of 100,000 units.
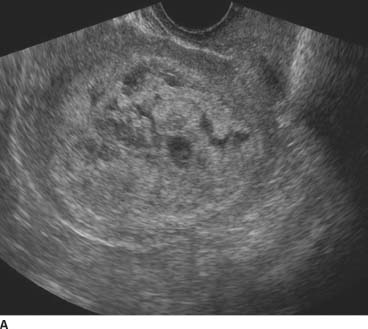
 FIGURE 9-1A Sagittal transvaginal gray-scale US scan of the uterus shows a large echogenic mass with multiple hypoechoic foci distending the endometrial cavity.
FIGURE 9-1A Sagittal transvaginal gray-scale US scan of the uterus shows a large echogenic mass with multiple hypoechoic foci distending the endometrial cavity.
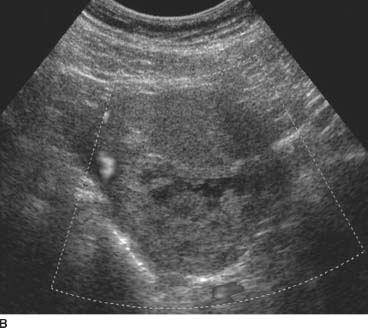
 FIGURE 9-1B Corresponding color Doppler ultrasound image demonstrates absence of flow within the cystic spaces. (See color insert)
FIGURE 9-1B Corresponding color Doppler ultrasound image demonstrates absence of flow within the cystic spaces. (See color insert)
 Gestational trophoblastic disease or complete molar pregnancy: The uterus is enlarged and the endometrial canal is filled with echogenic material. Anechoic areas within the endometrium do not fill with color consistent with cystic spaces. This would be the most likely diagnosis given the sonographic findings and P-HCG.
Gestational trophoblastic disease or complete molar pregnancy: The uterus is enlarged and the endometrial canal is filled with echogenic material. Anechoic areas within the endometrium do not fill with color consistent with cystic spaces. This would be the most likely diagnosis given the sonographic findings and P-HCG.
 Partial molar pregnancy: The imaging characteristics of a partial mole can be very similar to a complete molar pregnancy or abortion; however, in a partial molar pregnancy, a gestational sac or fetal tissue is present. The gestational sac and yolk sac are frequently abnormal and fetal anomalies are present. No evidence of an IUP was present in this case.
Partial molar pregnancy: The imaging characteristics of a partial mole can be very similar to a complete molar pregnancy or abortion; however, in a partial molar pregnancy, a gestational sac or fetal tissue is present. The gestational sac and yolk sac are frequently abnormal and fetal anomalies are present. No evidence of an IUP was present in this case.
 Hydropic degeneration of the placenta: This can occur in a first trimester abortion but the β-HCG will be lower than in a molar pregnancy and will trend downwards.
Hydropic degeneration of the placenta: This can occur in a first trimester abortion but the β-HCG will be lower than in a molar pregnancy and will trend downwards.
 Ectopic pregnancy: A decidual reaction in the setting of an ectopic pregnancy can result in a thickened, echogenic endometrium. Careful evaluation of the adnexa should be performed in any patient with a positive β-HCG.
Ectopic pregnancy: A decidual reaction in the setting of an ectopic pregnancy can result in a thickened, echogenic endometrium. Careful evaluation of the adnexa should be performed in any patient with a positive β-HCG.
DIAGNOSIS
Gestational trophoblastic disease (molar pregnancy)
KEY FACTS
Clinical
 Vaginal bleeding is the most common presentation. Other symptoms include hyperemesis gravidarum, hyperthyroidism, and toxemia. On physical exam, the uterus is larger than expected for dates.
Vaginal bleeding is the most common presentation. Other symptoms include hyperemesis gravidarum, hyperthyroidism, and toxemia. On physical exam, the uterus is larger than expected for dates.
 Risk factors include advancing maternal age, prior history of a molar pregnancy, and Asian ancestry.
Risk factors include advancing maternal age, prior history of a molar pregnancy, and Asian ancestry.
 Management is suction and curettage. Pathologic evaluation of the placenta demonstrates hydropic villi and trophoblastic proliferation.
Management is suction and curettage. Pathologic evaluation of the placenta demonstrates hydropic villi and trophoblastic proliferation.
 There is a 20% incidence of associated malignancy and surveillance is performed with serial P-HCG. Contraception for at least a year is recommended.
There is a 20% incidence of associated malignancy and surveillance is performed with serial P-HCG. Contraception for at least a year is recommended.
 Twin pregnancies have been reported with a coexisting normal fetus and placenta.
Twin pregnancies have been reported with a coexisting normal fetus and placenta.
Radiologic
 Distinguishing a complete mole, partial mole, and hydropic degeneration of the placenta can be difficult sonographically. The clinical presentation and serum β-HCG are important in making the correct diagnosis.
Distinguishing a complete mole, partial mole, and hydropic degeneration of the placenta can be difficult sonographically. The clinical presentation and serum β-HCG are important in making the correct diagnosis.
 The endometrial canal is distended with multiple tiny, hypoechoic spaces. Early in pregnancy, the only finding may be echogenic material within the endometrial canal. Cystic spaces develop as the placental villi degenerate.
The endometrial canal is distended with multiple tiny, hypoechoic spaces. Early in pregnancy, the only finding may be echogenic material within the endometrial canal. Cystic spaces develop as the placental villi degenerate.
 There is incomplete or inadequate vascularization and evaluation with color Doppler demonstrates decreased flow.
There is incomplete or inadequate vascularization and evaluation with color Doppler demonstrates decreased flow.
 Theca lutein cysts are present in up to 50% of molar pregnancies. They occur later in gestation and are thought to be secondary to the elevated β-HCG levels.
Theca lutein cysts are present in up to 50% of molar pregnancies. They occur later in gestation and are thought to be secondary to the elevated β-HCG levels.
SUGGESTED READING
Dighe M, Cuevas C, Moshiri M, et al. Sonography in first trimester bleeding. J Clin Ultrasound 2008;36:352–366.
Fraser-Hill MA, Wilson SR. Gestational trophoblastic neoplasia. In CM Rumack, SR Wilson, JW Charbonneau (eds). Diagnostic Ultrasound (3rd ed) (Vol 1). 2004; 589–601.
Green CL, Angtuaco TL, Shah HR, Parmley TH. Gestational trophoblastic disease: A spectrum of radiologic diagnosis. Radiographics 1996;16:1371–1384.
Lazarus E, Hulka C, Seiwert B, Levine D. Sonographic appearance of early complete molar pregnancies. J Ultrasound Med 1999;18:589–594.
Nalaboff KM, Pellerito S, Ben-Levi E. Imaging the endometrium: Disease and normal variants. Radiographics 2001;21:1409–1424.
JAMES D.
BOWIE
HISTORY
A 32-year-old pregnant woman who by examination is “large for dates.”
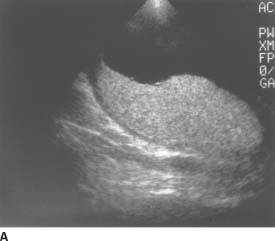
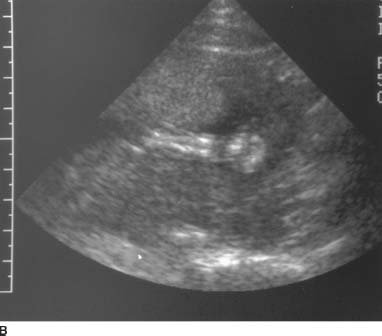
 FIGURE 9-2A Longitudinal sonogram of the placenta. There is slight thickening of the placenta, with a large pocket of amniotic fluid.
FIGURE 9-2A Longitudinal sonogram of the placenta. There is slight thickening of the placenta, with a large pocket of amniotic fluid.
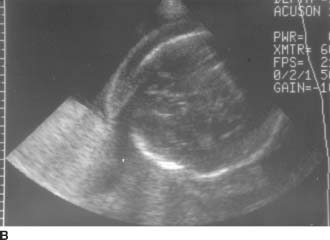
 FIGURE 9-2B Transverse sonogram of the fetal head. There is marked thickening of the skin over the fetal head.
FIGURE 9-2B Transverse sonogram of the fetal head. There is marked thickening of the skin over the fetal head.
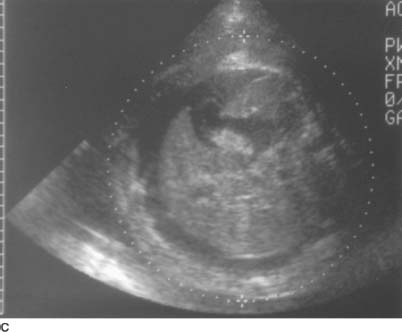
 FIGURE 9-2C Transverse sonogram of the fetal body. There is fetal ascites outlining the liver and spleen. There is also skin edema.
FIGURE 9-2C Transverse sonogram of the fetal body. There is fetal ascites outlining the liver and spleen. There is also skin edema.
 Placental enlargement: This is one manifestation of hydrops. It is also seen with intrauterine infections and certain chromosomal abnormalities (triploidy most commonly) and in some patients with diabetes.
Placental enlargement: This is one manifestation of hydrops. It is also seen with intrauterine infections and certain chromosomal abnormalities (triploidy most commonly) and in some patients with diabetes.
 Polyhydramnios: This is another manifestation of hydrops. It is also seen in a large number of conditions, including twins, diabetic mothers, and various fetal abnormalities including high gastrointestinal obstructions, central nervous system abnormalities, cardiac abnormalities (both structural and arrhythmias), and in a host of miscellaneous conditions including certain types of limb-shortening syndromes. Many cases of polyhydramnios have no known cause.
Polyhydramnios: This is another manifestation of hydrops. It is also seen in a large number of conditions, including twins, diabetic mothers, and various fetal abnormalities including high gastrointestinal obstructions, central nervous system abnormalities, cardiac abnormalities (both structural and arrhythmias), and in a host of miscellaneous conditions including certain types of limb-shortening syndromes. Many cases of polyhydramnios have no known cause.
 Skin edema: This is a common feature of hydrops. A similar appearance is seen in lymphangiectasia, which is often present in association with cystic hygromas. Infants of diabetic mothers with severe macrosomia are often mistaken for babies with skin edema.
Skin edema: This is a common feature of hydrops. A similar appearance is seen in lymphangiectasia, which is often present in association with cystic hygromas. Infants of diabetic mothers with severe macrosomia are often mistaken for babies with skin edema.
 Fluid collections: Collections of fluid within the fetus are frequently seen as an indicator of hydrops. Isolated fluid collections can be seen as a result of local inflammation, obstruction, or unknown mechanisms in conditions other than hydrops. For example, obstruction of the thoracic duct can lead to pleural fluid, and meconium peritonitis can lead to peritoneal fluid, as can rupture of an obstructed bladder or ureter.
Fluid collections: Collections of fluid within the fetus are frequently seen as an indicator of hydrops. Isolated fluid collections can be seen as a result of local inflammation, obstruction, or unknown mechanisms in conditions other than hydrops. For example, obstruction of the thoracic duct can lead to pleural fluid, and meconium peritonitis can lead to peritoneal fluid, as can rupture of an obstructed bladder or ureter.
DIAGNOSIS
Hydrops fetalis of undetermined etiology
KEY FACTS
Clinical
 Hydrops fetalis is a condition in the fetus characterized by accumulation of fluid, or edema, in at least two fetal compartments.
Hydrops fetalis is a condition in the fetus characterized by accumulation of fluid, or edema, in at least two fetal compartments.
 Most hydrops is still a result of an immune reaction between the fetus and the mother. Rh incompatibility is no longer the most common cause of these in Western nations.
Most hydrops is still a result of an immune reaction between the fetus and the mother. Rh incompatibility is no longer the most common cause of these in Western nations.
 Rhogam is given routinely to Rh-negative mothers in situations of potential Rh exposure.
Rhogam is given routinely to Rh-negative mothers in situations of potential Rh exposure.
 Alpha-thalassemia is an important cause of hydrops in Asia.
Alpha-thalassemia is an important cause of hydrops in Asia.
 Nonimmune fetal hydrops (NIFH) has a 50% to 95% mortality.
Nonimmune fetal hydrops (NIFH) has a 50% to 95% mortality.
 Clinical workup for hydrops fetalis includes blood type and Rh, antibody screen, VDRL, Kleihauer-Betke test for fetal cells, acute and convalescent TORCH titers, and, in some cases, maternal hemoglobin electrophoresis.
Clinical workup for hydrops fetalis includes blood type and Rh, antibody screen, VDRL, Kleihauer-Betke test for fetal cells, acute and convalescent TORCH titers, and, in some cases, maternal hemoglobin electrophoresis.
 Careful sonographic examination of the fetus, cord, and placenta is essential.
Careful sonographic examination of the fetus, cord, and placenta is essential.
Radiologic
 The diagnosis of hydrops is made when some combination of the following findings are observed: placental thickening, polyhydramnios, dilatation of the umbilical vein, pericardial fluid, pleural fluid, ascites, and subcutaneous edema. It is not clear what the earliest indicator of hydrops is or how many or which signs are the most specific. In general, if any one sign is present, early hydrops should be in the differential diagnosis unless there is another well-documented explanation for the abnormality. When three or more of the findings are present, it is highly likely that hydrops is the appropriate diagnosis.
The diagnosis of hydrops is made when some combination of the following findings are observed: placental thickening, polyhydramnios, dilatation of the umbilical vein, pericardial fluid, pleural fluid, ascites, and subcutaneous edema. It is not clear what the earliest indicator of hydrops is or how many or which signs are the most specific. In general, if any one sign is present, early hydrops should be in the differential diagnosis unless there is another well-documented explanation for the abnormality. When three or more of the findings are present, it is highly likely that hydrops is the appropriate diagnosis.
 The task of the sonologist in hydrops is to either guide for percutaneous umbilical blood sampling (PUBS) or to investigate the intrauterine contents carefully for an identifiable cause of NIFH. NIFH has too many causes to list in detail, and hence careful examination of the entire intrauterine contents is necessary. A rough grouping of these conditions is as follows:
The task of the sonologist in hydrops is to either guide for percutaneous umbilical blood sampling (PUBS) or to investigate the intrauterine contents carefully for an identifiable cause of NIFH. NIFH has too many causes to list in detail, and hence careful examination of the entire intrauterine contents is necessary. A rough grouping of these conditions is as follows:
 Structural cardiac defects. Also consider trisomy 13 and 18 if these are seen.
Structural cardiac defects. Also consider trisomy 13 and 18 if these are seen.
 Fetal arrhythmia. Either very fast or very slow. Look for arteriovenous block and consider an autoimmune process in the mother.
Fetal arrhythmia. Either very fast or very slow. Look for arteriovenous block and consider an autoimmune process in the mother.
 Fetal infections. These are usually detected by serum titers, but focal fluid collections and abdominal calcifications might suggest this as a possibility.
Fetal infections. These are usually detected by serum titers, but focal fluid collections and abdominal calcifications might suggest this as a possibility.
 Peripheral shunts. (1) Placental tumors such as chorioangiomas, if near the cord insertion or large, may cause fetal hydrops. (2) Vein of Galen aneurysm, when large, may be associated with hydrops. (3) Acardiac twin. In this rare condition, one fetus supplies blood to a partial twin via placental vessels.
Peripheral shunts. (1) Placental tumors such as chorioangiomas, if near the cord insertion or large, may cause fetal hydrops. (2) Vein of Galen aneurysm, when large, may be associated with hydrops. (3) Acardiac twin. In this rare condition, one fetus supplies blood to a partial twin via placental vessels.
 Masses restricting blood return to the heart. (1) Cystic adenomatoid malformation of the fetal lung. (2) Mediastinal teratoma.
Masses restricting blood return to the heart. (1) Cystic adenomatoid malformation of the fetal lung. (2) Mediastinal teratoma.
SUGGESTED READING
Bellini C, Hennekam RC, Fulcheri E, et al. Etiology of nonimmune hydrops fetalis: A systematic review. Am J Med Genet A 2009;149A;844–851.
Fleischer AC, Killam AP, Boehm FH, et al. Hydrops fetalis: Sonographic evaluation and clinical implications. Radiology 1981;141:163–168.
Hoddick WK, Mahony BS, Callen PW, Filly RA. Placental thickness. J Ultrasound Med 1985;4:479–482.
Marino T. Ultrasound abnormalities of the amniotic fluid, membranes, umbilical cord, and placenta. Obstet Gynecol Clin North Am 2004;31:177–200.
JAMES D.
BOWIE
HISTORY
A 29-year-old pregnant woman who by examination is small for gestational age.
 FIGURES 9-3A and 9-3B A: Longitudinal sonogram of the fetal spine. B: Longitudinal sonogram of the fetal limb. In both figures, there is marked absence of amniotic fluid, with crowding of fetal parts, increased contact of fetal structuinition of the fetus.
FIGURES 9-3A and 9-3B A: Longitudinal sonogram of the fetal spine. B: Longitudinal sonogram of the fetal limb. In both figures, there is marked absence of amniotic fluid, with crowding of fetal parts, increased contact of fetal structuinition of the fetus.
DIFFERENTIAL DIAGNOSIS
 Oligohydramnios: The appearance is characteristic for oligohydramnios with little else to consider. The real issue is the cause of the oligohydramnios.
Oligohydramnios: The appearance is characteristic for oligohydramnios with little else to consider. The real issue is the cause of the oligohydramnios.
 Abdominal pregnancy: One feature of a rare abdominal pregnancy is the lack of amniotic fluid. This is variable and can be simply a manifestation of the unusual distribution of amniotic fluid. More typically, the fetus is in an extended position and the fetal limbs are spread rather than crowded. The diagnosis in this condition is made by tracing the myometrium from the cervix.
Abdominal pregnancy: One feature of a rare abdominal pregnancy is the lack of amniotic fluid. This is variable and can be simply a manifestation of the unusual distribution of amniotic fluid. More typically, the fetus is in an extended position and the fetal limbs are spread rather than crowded. The diagnosis in this condition is made by tracing the myometrium from the cervix.
DIAGNOSIS
Oligohydramnios from premature rupture of membranes, not clinically suspected
KEY FACTS
Clinical
 Fetal renal function becomes the primary determinant of amniotic fluid volume at about the 18th week of pregnancy.
Fetal renal function becomes the primary determinant of amniotic fluid volume at about the 18th week of pregnancy.
 Normal amniotic fluid volume increases steadily until about 32 to 34 weeks of pregnancy, then decreases slightly until 42 weeks, then decreases more rapidly.
Normal amniotic fluid volume increases steadily until about 32 to 34 weeks of pregnancy, then decreases slightly until 42 weeks, then decreases more rapidly.
 The normal variability of amniotic fluid volume at any gestational age is high.
The normal variability of amniotic fluid volume at any gestational age is high.
 Amniotic fluid volume is approximately 125 to 300 mL at 16 weeks and 400 to 2,000 mL at 32 weeks.
Amniotic fluid volume is approximately 125 to 300 mL at 16 weeks and 400 to 2,000 mL at 32 weeks.
 Clinically, oligohydramnios is suspected if the fundal height is >4 cm less than expected or fails to grow appropriately by serial examination.
Clinically, oligohydramnios is suspected if the fundal height is >4 cm less than expected or fails to grow appropriately by serial examination.
 Clinically, oligohydramnios is defined as <300 mL of fluid at term.
Clinically, oligohydramnios is defined as <300 mL of fluid at term.
 Oligohydramnios in the second trimester has a poor prognosis and is associated with fetal pulmonary hypoplasia.
Oligohydramnios in the second trimester has a poor prognosis and is associated with fetal pulmonary hypoplasia.
 Causes for oligohydramnios include the following:
Causes for oligohydramnios include the following:
 Ruptured membranes: Perhaps the most common, but usually clinically apparent.
Ruptured membranes: Perhaps the most common, but usually clinically apparent.
 Fetal distress: Including IUGR, but oligohydramnios may appear before IUGR is detected and also as an independent indicator of fetal distress.
Fetal distress: Including IUGR, but oligohydramnios may appear before IUGR is detected and also as an independent indicator of fetal distress.
 Fetal death: Cardiac motion should be looked for in every case.
Fetal death: Cardiac motion should be looked for in every case.
 Postmaturity: Oligohydramnios does not diagnose this state but is confirmatory and indicates a possible poor outcome.
Postmaturity: Oligohydramnios does not diagnose this state but is confirmatory and indicates a possible poor outcome.
 Fetal renal abnormalities: These require that renal excretion into the amniotic fluid be reduced in volume or electrolyte content. The most common are complete bladder outlet obstruction, renal agenesis, obstruction of one kidney (usually a ureteropelvic junction obstruction [UPJ]), or multicystic dysplasia (MCDK) with an abnormal contralateral kidney (UPJ, MCDK, or agenesis).
Fetal renal abnormalities: These require that renal excretion into the amniotic fluid be reduced in volume or electrolyte content. The most common are complete bladder outlet obstruction, renal agenesis, obstruction of one kidney (usually a ureteropelvic junction obstruction [UPJ]), or multicystic dysplasia (MCDK) with an abnormal contralateral kidney (UPJ, MCDK, or agenesis).
Radiologic
 Diagnosis of oligohydramnios can be made by inspection. This subjective impression has been shown to be as good as objective measurements. The appearance of poor fetal definition, increased contact of the fetus with the uterine wall, crowding of fetal parts, and lack of fluid are consistent with this diagnosis.
Diagnosis of oligohydramnios can be made by inspection. This subjective impression has been shown to be as good as objective measurements. The appearance of poor fetal definition, increased contact of the fetus with the uterine wall, crowding of fetal parts, and lack of fluid are consistent with this diagnosis.
 Some observers have looked for the largest single pocket of fluid and have measured either the volume or the greatest vertical diameter. When the latter is <2 cm, oligohydramnios is present.
Some observers have looked for the largest single pocket of fluid and have measured either the volume or the greatest vertical diameter. When the latter is <2 cm, oligohydramnios is present.
 The amniotic fluid index is commonly used. This index measures the largest vertical diameter of the pockets of fluid in each of the four quadrants of the uterus and takes their sum. The number is looked up in a gestational age-adjusted table. Generally, any number P5 cm is consistent with oligohydramnios.
The amniotic fluid index is commonly used. This index measures the largest vertical diameter of the pockets of fluid in each of the four quadrants of the uterus and takes their sum. The number is looked up in a gestational age-adjusted table. Generally, any number P5 cm is consistent with oligohydramnios.
 The use of color Doppler in oligohydramnios before attempting to withdraw fluid will reveal that what appears to be a small pocket of fluid in many cases is actually the umbilical cord.
The use of color Doppler in oligohydramnios before attempting to withdraw fluid will reveal that what appears to be a small pocket of fluid in many cases is actually the umbilical cord.
 A phenomenon called first-trimester oligohydramnios has been described. This is a misnomer. The term refers to a gestational sac that is small for the occupying embryo and suggests a poor prognosis.
A phenomenon called first-trimester oligohydramnios has been described. This is a misnomer. The term refers to a gestational sac that is small for the occupying embryo and suggests a poor prognosis.
SUGGESTED READING
Bromley B, Harlow BL, Laboda LA, et al. Small sac size in the first trimester: A predictor of poor fetal outcome. Radiology 1991;178:375–377.
Goldstein RB, Filly RA. Sonographic estimation of amniotic fluid volume: Subjective assessment versus pocket measurements. J Ultrasound Med 1988;7:363–369.
Marino, T. Ultrasound abnormalities of the amniotic fluid, membranes, umbilical cord, and placenta. Obstet Gynecol Clin North Am 2004;31:177–200.
Moore TR, Cayle JE. The amniotic fluid index in normal human pregnancy. Am J Obstet Gynecol 1990;162:1168–1173.
Queenan JT, Thompson W, Whitfield CR, et al. Amniotic fluid volumes in normal pregnancies. Am J Obstet Gynecol 1972;114:34–38.
Seeds, AE. Current concepts of amniotic fluid dynamics. Am J Obstet Gynecol 1980;138:575–586.
Schrimmer, DB Moore, TR Sonographic evaluation of amniotic fluid volume. Clin Obstet Gynecol 2002;45:1026–1038.
JAMES D.
BOWIE
HISTORY
A 29-year-old pregnant woman is referred for a routine fetal ultrasound.
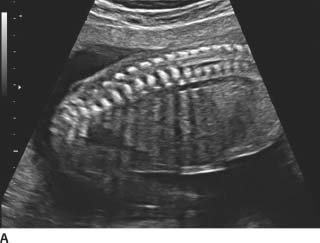
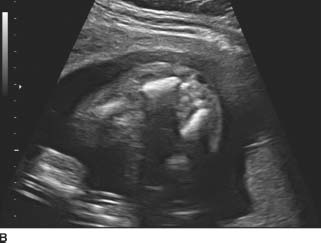
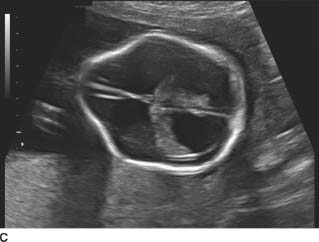
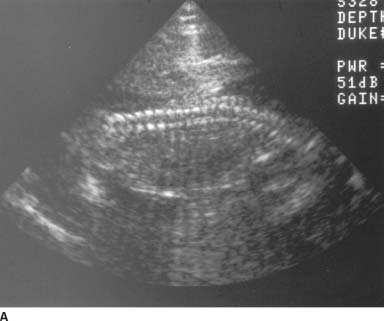
 FIGURE 9-4A, 9-4B, and 9-4C Sagittal (A) and transverse (B) gray-scale sonographic images of the fetal lumbosacral region show a small lumbar meningomyelocele. C : Transverse gray-scale US image of the fetal head shows scalloping of the frontal bones (the so-called, lemon sign) and ventriculomegaly. Note the dangling choroid plexus protruding into the dilated ventricles.
FIGURE 9-4A, 9-4B, and 9-4C Sagittal (A) and transverse (B) gray-scale sonographic images of the fetal lumbosacral region show a small lumbar meningomyelocele. C : Transverse gray-scale US image of the fetal head shows scalloping of the frontal bones (the so-called, lemon sign) and ventriculomegaly. Note the dangling choroid plexus protruding into the dilated ventricles.
 Chiari II malformation: This is the most likely diagnosis given the findings. A small sacral myelomeningocele is present, which should prompt for evaluation of the presence of Chiari II and associated ventriculomegaly. The frontal bones have a “lemon”configuration; however, this can be seen in normal pregnancies.
Chiari II malformation: This is the most likely diagnosis given the findings. A small sacral myelomeningocele is present, which should prompt for evaluation of the presence of Chiari II and associated ventriculomegaly. The frontal bones have a “lemon”configuration; however, this can be seen in normal pregnancies.
 Meningocele: This is the likely reason for the small echogenic focus at the distal spine, but a skin lesion such as a hemangioma could have a similar appearance.
Meningocele: This is the likely reason for the small echogenic focus at the distal spine, but a skin lesion such as a hemangioma could have a similar appearance.
 Sacrococcygeal teratomas: These may occur in the distal spine, but they are not directly posterior since they originate anterior to the spine. Furthermore, they tend to be much larger.
Sacrococcygeal teratomas: These may occur in the distal spine, but they are not directly posterior since they originate anterior to the spine. Furthermore, they tend to be much larger.
DIAGNOSIS
Chiari II malformation with a small sacral meningocele
KEY FACTS
Clinical
 Chiari II malformation is characterized by a small posterior fossa with downward displacement of the brain-stem, resulting in protrusion of the tonsils and vermis below the cisterna magna.
Chiari II malformation is characterized by a small posterior fossa with downward displacement of the brain-stem, resulting in protrusion of the tonsils and vermis below the cisterna magna.
 Secondary features seen prenatally include a meningocele or encephalocele in about 90% of cases and partial or complete absence of the corpus callosum in about 40% of cases.
Secondary features seen prenatally include a meningocele or encephalocele in about 90% of cases and partial or complete absence of the corpus callosum in about 40% of cases.
 Hydrocephalus is commonly seen by sonography as the first indication of Chiari II, but it is not always present early. The presence of ventriculomegaly is much more common after 24 weeks of gestation.
Hydrocephalus is commonly seen by sonography as the first indication of Chiari II, but it is not always present early. The presence of ventriculomegaly is much more common after 24 weeks of gestation.
 Many of the computed tomography (CT) or magnetic resonance imaging (MRI) features of this condition, such as a large massa intermedia, polymicrogyria, beaking of the tectal plate, hydromelia, and syringomelia, have not been described as prenatal sonographic features of Chiari II.
Many of the computed tomography (CT) or magnetic resonance imaging (MRI) features of this condition, such as a large massa intermedia, polymicrogyria, beaking of the tectal plate, hydromelia, and syringomelia, have not been described as prenatal sonographic features of Chiari II.
 Periconceptional intake of 0.4 mg of folic acid significantly reduces the risk of neural tube defects.
Periconceptional intake of 0.4 mg of folic acid significantly reduces the risk of neural tube defects.
 The overall prognosis for a myelomeningocele diagnosed in utero depends on the presence and type of other abnormalities. Approximately 50% to 80% of fetuses have other abnormalities.
The overall prognosis for a myelomeningocele diagnosed in utero depends on the presence and type of other abnormalities. Approximately 50% to 80% of fetuses have other abnormalities.
 Most deaths in patients with an isolated myelomeningocele are the result of the hindbrain dysfunction associated with Chiari II malformation. About 15% of patients die by the age of 10.
Most deaths in patients with an isolated myelomeningocele are the result of the hindbrain dysfunction associated with Chiari II malformation. About 15% of patients die by the age of 10.
 About 30% of survivors have IQs >100. About 30% have IQs <80. About one-third have hindbrain dysfunction.
About 30% of survivors have IQs >100. About 30% have IQs <80. About one-third have hindbrain dysfunction.
 Ninety percent of neonatal survivors require ventriculoperitoneal shunts. About half of these shunts must be revised by age 6. About a third require two shunt revisions and a fifth three or more.
Ninety percent of neonatal survivors require ventriculoperitoneal shunts. About half of these shunts must be revised by age 6. About a third require two shunt revisions and a fifth three or more.
 Almost 100% of survivors have some loss of motor function. Urinary incontinence is common.
Almost 100% of survivors have some loss of motor function. Urinary incontinence is common.
Radiologic
 The lemon sign has been said to be a specific finding of Chiari II malformations, but it is also seen in normals. This is especially true for milder degrees of frontal bone contour abnormalities.
The lemon sign has been said to be a specific finding of Chiari II malformations, but it is also seen in normals. This is especially true for milder degrees of frontal bone contour abnormalities.
 Infratentorial findings of Chiari II include the “banana”cerebellum and effacement of the cisterna magna. The degree of severity of the posterior fossa deformity correlates with the degree of hydrocephaly.
Infratentorial findings of Chiari II include the “banana”cerebellum and effacement of the cisterna magna. The degree of severity of the posterior fossa deformity correlates with the degree of hydrocephaly.
 Both the degree and prevalence of hydrocephaly increases with gestational age.
Both the degree and prevalence of hydrocephaly increases with gestational age.
 The absence of ventriculomegaly prior to 24 weeks does not exclude either a meningocele or Chiari II.
The absence of ventriculomegaly prior to 24 weeks does not exclude either a meningocele or Chiari II.
 Other sonographic findings have been described more recently in Chiari II including the ventricular “point,”abnormal tectal morphology, and the presence of an interhemispheric cyst.
Other sonographic findings have been described more recently in Chiari II including the ventricular “point,”abnormal tectal morphology, and the presence of an interhemispheric cyst.
SUGGESTED READING
Babcock CJ, Goldstein RB, Barth RA, et al. Prevalence of ventriculomegaly in association with myelomeningocele and severity of posterior fossa deformity. Radiology 1994;190:703–707.
Callen AL, Filly RA. Supratentorial abnormalities in the Chiari II malformation, I: The ventricular point. J Ultrasound Med 2008;27:33–38.
Callen AL, Stengel JW, Filly RA. Supratentorial abnormalities in the Chiari II malformation, II: Tectal morphologic changes. J Ultrasound Med 2009;28:29–35.
Filly RA. The “lemon”sign: A clinical perspective. Radiology 1998;167:573–575.
Wong SK, Barkovich AJ, Callen AL, Filly RA. Supratentorial abnormalities in the Chiari II malformation, III: The interhemispheric cyst. J Ultrasound Med 2009;28:999–1006.
CLARE M.
HAYSTEAD
HISTORY
A 54-year-old who underwent CT evaluation for vague abdominal pain. An ultrasound was recommended for further evaluation of the gallbladder.
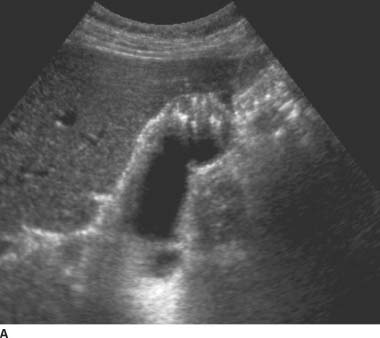
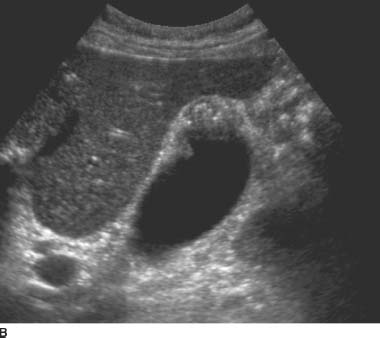
 FIGURES 9-5A and 9-5B Longitudinal gray-scale US images obtained with a 5.0-MHz curvilinear-array transducer demonstrate focal thickening of the gallbladder fundus with echogenic intramural foci and associated “comet tail”reverberation artifacts.
FIGURES 9-5A and 9-5B Longitudinal gray-scale US images obtained with a 5.0-MHz curvilinear-array transducer demonstrate focal thickening of the gallbladder fundus with echogenic intramural foci and associated “comet tail”reverberation artifacts.
 Primary gallbladder malignancy: Adenocarcinoma can present as irregular wall thickening, both focal and diffuse. The most common presentation is a mass replacing the gallbladder in the gallbladder fossa. Associated findings include wall calcification, biliary ductal dilatation, gallstones, and the presence of metastatic disease, especially liver involvement.
Primary gallbladder malignancy: Adenocarcinoma can present as irregular wall thickening, both focal and diffuse. The most common presentation is a mass replacing the gallbladder in the gallbladder fossa. Associated findings include wall calcification, biliary ductal dilatation, gallstones, and the presence of metastatic disease, especially liver involvement.
 Adenomyomatosis: The classic appearance is focal wall thickening in the gallbladder fundus. The presence of cystic spaces and echogenic foci with “comet tail”reverberation artifact is diagnostic of adenomyomatosis.
Adenomyomatosis: The classic appearance is focal wall thickening in the gallbladder fundus. The presence of cystic spaces and echogenic foci with “comet tail”reverberation artifact is diagnostic of adenomyomatosis.
 Emphysematous cholecystitis: Air within the gallbladder wall can cause echogenic foci with ring down artifacts. The gallbladder lumen is difficult to identify in diffuse emphysematous cholecystitis and patients are usually acutely symptomatic.
Emphysematous cholecystitis: Air within the gallbladder wall can cause echogenic foci with ring down artifacts. The gallbladder lumen is difficult to identify in diffuse emphysematous cholecystitis and patients are usually acutely symptomatic.
 Acute cholecystitis: Gallbladder wall thickening is one of the findings described in acute cholecystitis; however, the “comet tail”reverberation artifact in this case is diagnostic of adenomyomatosis.
Acute cholecystitis: Gallbladder wall thickening is one of the findings described in acute cholecystitis; however, the “comet tail”reverberation artifact in this case is diagnostic of adenomyomatosis.
DIAGNOSIS
Adenomyomatosis
KEY FACTS
Clinical
 Adenomyomatosis is characterized by benign hyper-plasia of the gallbladder mucosa that creates invaginations through the thickened muscular layer known as Rokitansky-Aschoff sinuses.
Adenomyomatosis is characterized by benign hyper-plasia of the gallbladder mucosa that creates invaginations through the thickened muscular layer known as Rokitansky-Aschoff sinuses.
 It is thought to be acquired and somewhat similar to colonic diverticulosis. Most patients are asymptomatic. Those that are symptomatic typically undergo a non-emergent cholecystectomy.
It is thought to be acquired and somewhat similar to colonic diverticulosis. Most patients are asymptomatic. Those that are symptomatic typically undergo a non-emergent cholecystectomy.
 Ninety percent have associated gallstones; however, the association is unclear.
Ninety percent have associated gallstones; however, the association is unclear.
 It is considered one of the hyperplastic cholecystosis, the other being cholesterolosis. As the name implies, it is a benign process.
It is considered one of the hyperplastic cholecystosis, the other being cholesterolosis. As the name implies, it is a benign process.
Radiologic
 Adenomyomatosis can cause focal, segmental, or diffuse wall thickening. Focal adenomyomatosis tends to be fundal in location as in this case. An “hourglass”shaped gallbladder can be seen in the segmental subtype resulting in thickening and narrowing of the midportion of the gallbladder wall.
Adenomyomatosis can cause focal, segmental, or diffuse wall thickening. Focal adenomyomatosis tends to be fundal in location as in this case. An “hourglass”shaped gallbladder can be seen in the segmental subtype resulting in thickening and narrowing of the midportion of the gallbladder wall.
 Multiple cystic spaces are present representing enlarged Rokitansky-Aschoff sinuses that are filled with bile.
Multiple cystic spaces are present representing enlarged Rokitansky-Aschoff sinuses that are filled with bile.
 Echogenic foci within the sinuses are thought to represent cholesterol deposits or small stones.
Echogenic foci within the sinuses are thought to represent cholesterol deposits or small stones.
 This condition can appear as focal gallbladder wall thickening on CT and differentiating it from gallbladder cancer can be difficult. The presence of intramural diverticuli increases diagnostic confidence.
This condition can appear as focal gallbladder wall thickening on CT and differentiating it from gallbladder cancer can be difficult. The presence of intramural diverticuli increases diagnostic confidence.
 The “pearl necklace”sign has been described on MRCP corresponding to curvilinear foci of high T2 signal within the gallbladder wall. Stones can form within the diverticuli causing signal voids on MRI.
The “pearl necklace”sign has been described on MRCP corresponding to curvilinear foci of high T2 signal within the gallbladder wall. Stones can form within the diverticuli causing signal voids on MRI.
SUGGESTED READING
Boscak AR, Al-hawary M, Ramsburgh SR. Best cases from the AFIP: Adenomyomatosis of the gallbladder. Radiographics 2006;26:941–946.
Ching BH, Yeh BM, Westphalen AC, et al. CT differentiation of adenomyomatosis and gallbladder cancer. AJR Am J Roentgenol 2007;182:62–66.
Ghersin E, Soudack M, Gaitini D. Twinkling artifact in gallbladder adenomyomatosis. J Ultrasound Med 2003;22:229–231.
Secil M, Karasu S, Sagol O, Coker A. Combined segmental and focal adenomyomatosis involving the body of the gallbladder. J Clin Ultrasound 2005;33:248–250.
Yoon, JH, Cha SS, Han SS, et al. Gallbladder adenomyomatosis: Imaging findings. Abdom Imaging 2006;31:555–563.
MARK A.
KLIEWER
HISTORY
A 35-year-old woman presents with vague right upper quadrant pain.
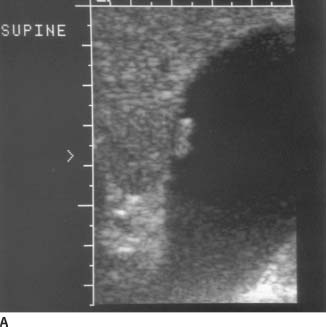
 FIGURE 9-6A Longitudinal sonogram of the gallbladder fossa. Attached to the gallbladder wall is a small echogenic mass. This mass does not cause significant ring down or shadowing artifacts.
FIGURE 9-6A Longitudinal sonogram of the gallbladder fossa. Attached to the gallbladder wall is a small echogenic mass. This mass does not cause significant ring down or shadowing artifacts.
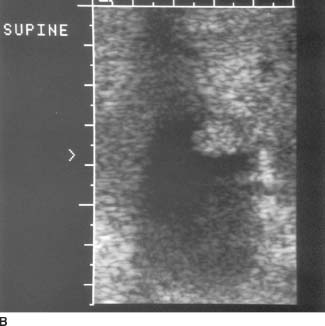
 FIGURE 9-6B High-resolution sonogram of this same location. High-resolution image reveals the lobular contour of the gallbladder wall mass.
FIGURE 9-6B High-resolution sonogram of this same location. High-resolution image reveals the lobular contour of the gallbladder wall mass.
 Gallstone: A gallstone is unlikely because the abnormality is adherent to the wall and nonmobile, and because of the absence of posterior acoustic shadowing.
Gallstone: A gallstone is unlikely because the abnormality is adherent to the wall and nonmobile, and because of the absence of posterior acoustic shadowing.
 Cholesterol polyp: These polyps are common intraluminal masses that are most often multiple. The polyps tend to be small, usually <5 mm in size, and almost always <1 cm in size. They are not associated with acoustic shadowing.
Cholesterol polyp: These polyps are common intraluminal masses that are most often multiple. The polyps tend to be small, usually <5 mm in size, and almost always <1 cm in size. They are not associated with acoustic shadowing.
 Adenoma or papilloma: These lesions are almost always singular and are much less common than cholesterol polyps. These masses can be sessile and broad based (adenomas) or pedunculated and lobulated (papillary adenomas). Masses are usually <1 cm.
Adenoma or papilloma: These lesions are almost always singular and are much less common than cholesterol polyps. These masses can be sessile and broad based (adenomas) or pedunculated and lobulated (papillary adenomas). Masses are usually <1 cm.
 Primary gallbladder carcinoma: Carcinomas are most often seen as a large, solid mass replacing the gallbladder in the gallbladder fossa. Occasionally, in earlier stages focal intraluminal masses are seen, but these are usually >1 cm in size. Associated findings include wall calcification, biliary duct dilatation, gallstones (80%), and evidence for metastatic spread, particularly to the liver.
Primary gallbladder carcinoma: Carcinomas are most often seen as a large, solid mass replacing the gallbladder in the gallbladder fossa. Occasionally, in earlier stages focal intraluminal masses are seen, but these are usually >1 cm in size. Associated findings include wall calcification, biliary duct dilatation, gallstones (80%), and evidence for metastatic spread, particularly to the liver.
 Tumefactive sludge: This echogenic bile is not usually adherent to the wall but rather shifts location as the patient is repositioned.
Tumefactive sludge: This echogenic bile is not usually adherent to the wall but rather shifts location as the patient is repositioned.
DIAGNOSIS
Cholesterol polyp
KEY FACTS
Clinical
 Cholesterolosis results from the accumulation of cholesterol within the wall of the gallbladder. This accumulation can either be diffuse or polypoid.
Cholesterolosis results from the accumulation of cholesterol within the wall of the gallbladder. This accumulation can either be diffuse or polypoid.
 Cholesterol nodules can stud the mucosal surface, producing the so-called strawberry gallbladder seen pathologically.
Cholesterol nodules can stud the mucosal surface, producing the so-called strawberry gallbladder seen pathologically.
 Cholesterolosis is equally common in men and women. The etiology is uncertain.
Cholesterolosis is equally common in men and women. The etiology is uncertain.
Radiologic
 Intraluminal masses are commonly seen during gallbladder ultrasound. Fortunately, the majority of these are cholesterol polyps that are benign.
Intraluminal masses are commonly seen during gallbladder ultrasound. Fortunately, the majority of these are cholesterol polyps that are benign.
 Sonographically, cholesterol polyps are most commonly multiple and small. Most of these benign polypoid masses in the gallbladder are <1 cm. It is common clinical practice to follow polyps between 5 mm and 1 cm for a 2-year period to document any change or growth of the lesion. Lesions that are significantly >1 cm may require surgery.
Sonographically, cholesterol polyps are most commonly multiple and small. Most of these benign polypoid masses in the gallbladder are <1 cm. It is common clinical practice to follow polyps between 5 mm and 1 cm for a 2-year period to document any change or growth of the lesion. Lesions that are significantly >1 cm may require surgery.
 Cholesterol polyps are nonmobile intraluminal masses that are firmly adherent to the wall. They do not cause acoustic shadowing. Such polyps can be hypo-or hyperechoic, though most, in our experience, are hyperechoic.
Cholesterol polyps are nonmobile intraluminal masses that are firmly adherent to the wall. They do not cause acoustic shadowing. Such polyps can be hypo-or hyperechoic, though most, in our experience, are hyperechoic.
SUGGESTED READING
Collett JA, Allan RB, Chisholm RJ, et al. Gallbladder polyps: Prospective study. J Ultrasound Med 1998;17:207–211.
Jutras JA. Hyperplastic cholecystoses. AJR Am J Roentgenol 1960;83:795–827.
Levy AD, Murakata LA, Abbott RM, Rohrmann CA Jr. From the archives of the AFIP. Benign tumors and tumorlike lesions of the gallbladder and extrahepatic bile ducts: Radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics 2002;22:387–413.
Middleton WD. Gallbladder. In BB Goldberg (ed), Textbook of Abdominal Ultrasound. Baltimore: Williams & Wilkins, 1993;116–145.
Price RJ, Stewart ET, Foley WD, Dodds WJ. Sonography of polypoid cholesterolosis. AJR Am J Roentgenol 1982;139:1197–1198.
Ruhe AH, Zachman JP, Mulder BD, Rime AE. Cholesterol polyps of the gallbladder: Ultrasound demonstration. J Clin Ultrasound 1979;7:386–388.
MARK A.
KLIEWER
HISTORY
A 53-year-old man presents with acute abdominal pain and vomiting.
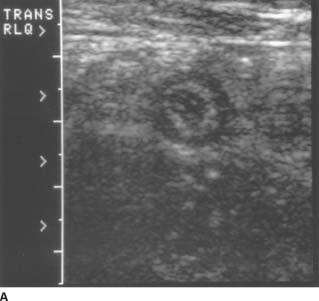
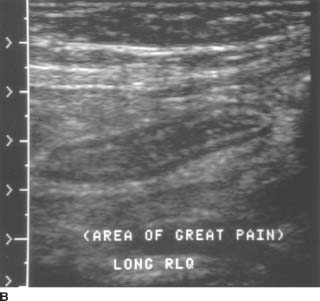
 FIGURES 9-7A and 9-7B A: Transverse sonogram of the right lower quadrant. B: Longitudinal sonogram of the same area in the right lower quadrant. The transducer was placed over the area of greatest tenderness. Longitudinal and transverse images demonstrate a noncompressible, tubular structure measuring 11 mm in diameter. The rounded end of the structure is clearly identifiable. The structure demonstrates a hyperechoic inner layer and a surrounding, peripheral hypoechoic layer.
FIGURES 9-7A and 9-7B A: Transverse sonogram of the right lower quadrant. B: Longitudinal sonogram of the same area in the right lower quadrant. The transducer was placed over the area of greatest tenderness. Longitudinal and transverse images demonstrate a noncompressible, tubular structure measuring 11 mm in diameter. The rounded end of the structure is clearly identifiable. The structure demonstrates a hyperechoic inner layer and a surrounding, peripheral hypoechoic layer.
 Normal appendix: The normal appendix should have a diameter of <6 mm and should be compressible. In addition, there should be no evidence of inflammatory changes in the periappendiceal fat.
Normal appendix: The normal appendix should have a diameter of <6 mm and should be compressible. In addition, there should be no evidence of inflammatory changes in the periappendiceal fat.
 Crohn’s disease: Hypoechoic, uniform thickening of the bowel wall in the terminal ileum would surround compressed echogenic mucosa centrally. Furthermore, the terminal ileum would not have a blind end.
Crohn’s disease: Hypoechoic, uniform thickening of the bowel wall in the terminal ileum would surround compressed echogenic mucosa centrally. Furthermore, the terminal ileum would not have a blind end.
 Appendicitis: This is the best diagnosis for a rigid tubular structure located in the right lower quadrant at the site of maximal tenderness. The diameter measurement from the outer wall to the outer wall of the structure exceeds 6 mm. The structure is noncompressible and blind ended. On the longitudinal view, a region of increased echogenicity surrounding the appendix suggests inflammation in the periappendiceal fat.
Appendicitis: This is the best diagnosis for a rigid tubular structure located in the right lower quadrant at the site of maximal tenderness. The diameter measurement from the outer wall to the outer wall of the structure exceeds 6 mm. The structure is noncompressible and blind ended. On the longitudinal view, a region of increased echogenicity surrounding the appendix suggests inflammation in the periappendiceal fat.
 Mesenteric adenitis: With this disease process, enlarged lymph nodes and mural thickening of the terminal ileum will be evident. Peristalsis should also be present in the terminal ileum. Inflamed lymph nodes would be unlikely to have such a smooth tubular contour, or the layering of hyperechoic and hypoechoic strata that constitute the gut signature.
Mesenteric adenitis: With this disease process, enlarged lymph nodes and mural thickening of the terminal ileum will be evident. Peristalsis should also be present in the terminal ileum. Inflamed lymph nodes would be unlikely to have such a smooth tubular contour, or the layering of hyperechoic and hypoechoic strata that constitute the gut signature.
 Pelvic inflammatory disease (PID): Though PID is a possible pitfall, findings should be located more in the pelvis than the right lower quadrant. A hydrosalpinx would not have the same echo pattern as the bowel.
Pelvic inflammatory disease (PID): Though PID is a possible pitfall, findings should be located more in the pelvis than the right lower quadrant. A hydrosalpinx would not have the same echo pattern as the bowel.
DIAGNOSIS
Appendicitis
KEY FACTS
Clinical
 Though the clinical suspicion of appendicitis is correct in 70% of patients with this disease, the clinical picture can be confusing. As a result, surgeons have long accepted a removal rate of normal appendices between 15% and 33%. This percentage has diminished, however, with the widespread use of multidetector CT.
Though the clinical suspicion of appendicitis is correct in 70% of patients with this disease, the clinical picture can be confusing. As a result, surgeons have long accepted a removal rate of normal appendices between 15% and 33%. This percentage has diminished, however, with the widespread use of multidetector CT.
 Young women can be particularly difficult to diagnose because of the similarity of symptoms between appendicitis, PID, and ovarian torsion.
Young women can be particularly difficult to diagnose because of the similarity of symptoms between appendicitis, PID, and ovarian torsion.
Radiologic
 The technique of examination requires using high-frequency, linear transducers (5 or 7 MHz). The sonologist applies graded compression with the transducer and moves from the right upper quadrant to the right lower quadrant following the right colon inferiorly. Alternatively, the iliac vessels can be identified in the groin and followed superiorly.
The technique of examination requires using high-frequency, linear transducers (5 or 7 MHz). The sonologist applies graded compression with the transducer and moves from the right upper quadrant to the right lower quadrant following the right colon inferiorly. Alternatively, the iliac vessels can be identified in the groin and followed superiorly.
 The patient can often direct attention to pathology by localizing pain. Be alert to areas of maximal tenderness.
The patient can often direct attention to pathology by localizing pain. Be alert to areas of maximal tenderness.
 The goal is to compress the inflamed appendix against the posterior abdominal wall or the psoas muscles.
The goal is to compress the inflamed appendix against the posterior abdominal wall or the psoas muscles.
 An inflamed appendix is noncompressible and has a diameter of >6 mm.
An inflamed appendix is noncompressible and has a diameter of >6 mm.
 A fecalith may be seen within the appendix and may be associated with acoustic shadowing.
A fecalith may be seen within the appendix and may be associated with acoustic shadowing.
 Look also for periappendiceal fluid, abscess, or an inflammatory mass. The circumferential echogenic mucosal layer of the appendix may be disrupted when perforation occurs. The periappendiceal fat may be echogenic, indicating inflammatory involvement.
Look also for periappendiceal fluid, abscess, or an inflammatory mass. The circumferential echogenic mucosal layer of the appendix may be disrupted when perforation occurs. The periappendiceal fat may be echogenic, indicating inflammatory involvement.
 Increased Doppler signal (blood flow) can be found both within and surrounding the inflamed appendix.
Increased Doppler signal (blood flow) can be found both within and surrounding the inflamed appendix.
SUGGESTED READING
Noguchi N, Yoshimitsu K, Yoshida M. Periappendiceal hyperechoic structure on sonography: A sign of severe appendicitis. J Ultrasound Med 2005;24:323–327.
Puylaert JB. Ultrasonography of the acute abdomen: Gastrointestinal conditions. Radiol Clin North Am 2003;41:1227–1242.
Rettenbacher T, Hollerweger A, Macheiner P, et al. Outer diameter of the vermiform appendix as a sign of acute appendicitis: Evaluation at US. Radiology 2001;218:757–762.
Worrell JA, Droishagen LF, Kelly TC, et al. Graded compression ultrasound in the diagnosis of appendicitis. J Ultrasound Med 1990;9:145–150.
MARK A.
KLIEWER
HISTORY
A 26-year-old woman presents for a routine obstetric ultrasound.
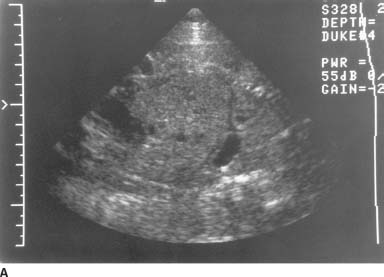
 FIGURE 9-8A Longitudinal sonogram of the fetal body, cephalic presentation. The fetal thorax is on the right of the image and the fetal abdomen on the left. The hypoechoic arc of the diaphragm is apparently disrupted. Superior to the diaphragm and posterior to the heart, there is a rounded, fluid-filled structure that most likely represents the stomach.
FIGURE 9-8A Longitudinal sonogram of the fetal body, cephalic presentation. The fetal thorax is on the right of the image and the fetal abdomen on the left. The hypoechoic arc of the diaphragm is apparently disrupted. Superior to the diaphragm and posterior to the heart, there is a rounded, fluid-filled structure that most likely represents the stomach.
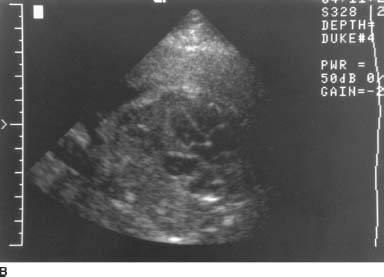
 FIGURE 9-8B Transverse sonogram through the fetal chest at the level of the heart demonstrates the fluid-filled structure adjacent to the heart. In this cephalic presentation, the heart is on the right side of the fetal body, indicating displacement from its normal position.
FIGURE 9-8B Transverse sonogram through the fetal chest at the level of the heart demonstrates the fluid-filled structure adjacent to the heart. In this cephalic presentation, the heart is on the right side of the fetal body, indicating displacement from its normal position.
 Cystic adenomatoid malformation: This entity is typically seen as multiple large cysts or an echogenic mass. Occasionally, it can occur with a diaphragmatic hernia.
Cystic adenomatoid malformation: This entity is typically seen as multiple large cysts or an echogenic mass. Occasionally, it can occur with a diaphragmatic hernia.
 Congenital diaphragmatic hernia (CDH): A hernia of this type is a likely possibility given the displacement of the stomach into the chest and the apparent discontinuity of the hypoechoic diaphragm.
Congenital diaphragmatic hernia (CDH): A hernia of this type is a likely possibility given the displacement of the stomach into the chest and the apparent discontinuity of the hypoechoic diaphragm.
 Bronchogenic and esophageal duplication cyst: It is possible that the fluid-filled structure in the left chest could be a foregut abnormality; however, the stomach is not present in its expected location.
Bronchogenic and esophageal duplication cyst: It is possible that the fluid-filled structure in the left chest could be a foregut abnormality; however, the stomach is not present in its expected location.
 Cystic teratoma: These are typically complex masses with cystic and solid components arising in the mediastinum.
Cystic teratoma: These are typically complex masses with cystic and solid components arising in the mediastinum.
 Eventration of the diaphragm: It is difficult to exclude eventration with certainty, considering how difficult it is to demonstrate the full contour of the diaphragm in the normal fetus.
Eventration of the diaphragm: It is difficult to exclude eventration with certainty, considering how difficult it is to demonstrate the full contour of the diaphragm in the normal fetus.
DIAGNOSIS
Congenital diaphragmatic hernia
KEY FACTS
Clinical
 Herniation of bowel or solid organs can occur through defects in the diaphragm that form from incomplete closure during embryologic development. Most are Bochdalek defects, which occur posteriorly, usually on the left side.
Herniation of bowel or solid organs can occur through defects in the diaphragm that form from incomplete closure during embryologic development. Most are Bochdalek defects, which occur posteriorly, usually on the left side.
 Bowel herniating through the left-sided defect will cause displacement of thoracic structures, particularly the heart, to the right.
Bowel herniating through the left-sided defect will cause displacement of thoracic structures, particularly the heart, to the right.
 When hernias occur on the right side, the liver may be involved.
When hernias occur on the right side, the liver may be involved.
 Patients sometimes present because their fundal size is greater than their dates. This results when there is associated polyhydramnios, which most often develops in the third trimester.
Patients sometimes present because their fundal size is greater than their dates. This results when there is associated polyhydramnios, which most often develops in the third trimester.
 More than half of babies with CDH will have an associated anomaly. These anomalies can involve the heart, genitourinary system, the central nervous system, and the gastrointestinal tract. The diaphragmatic hernia can be part of a larger syndrome, such as the trisomy syndromes.
More than half of babies with CDH will have an associated anomaly. These anomalies can involve the heart, genitourinary system, the central nervous system, and the gastrointestinal tract. The diaphragmatic hernia can be part of a larger syndrome, such as the trisomy syndromes.
 There is a high mortality rate for infants born with diaphragmatic hernias resulting primarily from pulmonary hypoplasia.
There is a high mortality rate for infants born with diaphragmatic hernias resulting primarily from pulmonary hypoplasia.
Radiologic
 Sonographic diagnosis depends on the demonstration of bowel in the fetal thorax. On a true transverse image, the heart should never be at the same level as the stomach. Occasionally, hepatic, portal, and mesenteric blood vessels will be displaced toward the defect. The stomach will not be located in its expected left upper quadrant position, and the abdomen can appear scaphoid due to the relocation of bowel from the abdomen to the thorax.
Sonographic diagnosis depends on the demonstration of bowel in the fetal thorax. On a true transverse image, the heart should never be at the same level as the stomach. Occasionally, hepatic, portal, and mesenteric blood vessels will be displaced toward the defect. The stomach will not be located in its expected left upper quadrant position, and the abdomen can appear scaphoid due to the relocation of bowel from the abdomen to the thorax.
 Intrathoracic structures are usually displaced to the right with left-sided lesions. The heart, in particular, will be found on the right side. Occasionally, the diaphragm can be inspected directly and a defect identified. The motion of the two hemidiaphragms can be compared during fetal breathing for evidence of dissynchronous motion.
Intrathoracic structures are usually displaced to the right with left-sided lesions. The heart, in particular, will be found on the right side. Occasionally, the diaphragm can be inspected directly and a defect identified. The motion of the two hemidiaphragms can be compared during fetal breathing for evidence of dissynchronous motion.
 Polyhydramnios frequently complicates the pregnancy.
Polyhydramnios frequently complicates the pregnancy.
 The sonologist should always look for other evidence of fetal abnormality, including other structural anomalies and intrauterine growth retardation.
The sonologist should always look for other evidence of fetal abnormality, including other structural anomalies and intrauterine growth retardation.
 MRI is used increasingly in the evaluation of CDH.
MRI is used increasingly in the evaluation of CDH.
SUGGESTED READING
Chinn DH, Filly RA, Callen PW, et al. Congenital diaphragmatic hernia diagnosed prenatally by ultrasound. Radiology 1983;148:119–123.
Comstock CH. The antenatal diagnosis of diaphragmatic anomalies. J Ultrasound Med 1986;5:391–396.
Leung JW. Prenatal MR imaging of congenital diaphragmatic hernia. AJR Am J Roentgenol 2000;174:1607–1612.
May DA, Barth RA, Yeager S, et al. Perinatal and postnatal chest sonography. Radiol Clin North Am 1993;31:499–516.
Morin L, Crombleholme TM, D’Alton ME. Prenatal diagnosis and management of fetal thoracic lesions. Semin Perinatol 1994;18:228–253.
Vettraino IM, Lee W, Comstock CH. The evolving appearance of a congenital diaphragmatic hernia. J Ultrasound Med 2002;21:85–89.
BARBARA S.
HERTZBERG
HISTORY
A 28-year-old woman has vaginal bleeding and a positive β-HCG level.
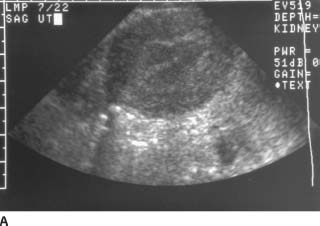
 FIGURE 9-9A Sagittal endovaginal sonogram of the uterus. There is no evidence of an intrauterine pregnancy. The endometrial stripe is normal.
FIGURE 9-9A Sagittal endovaginal sonogram of the uterus. There is no evidence of an intrauterine pregnancy. The endometrial stripe is normal.
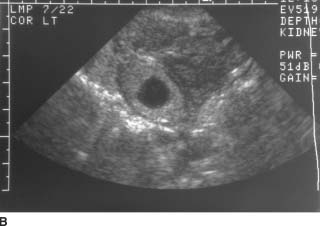
 FIGURE 9-9B Coronal endovaginal sonogram of the left adnexa. An echogenic ring surrounds a rounded fluid collection in the left adnexa, consistent with an “adnexal ring sign.”
FIGURE 9-9B Coronal endovaginal sonogram of the left adnexa. An echogenic ring surrounds a rounded fluid collection in the left adnexa, consistent with an “adnexal ring sign.”
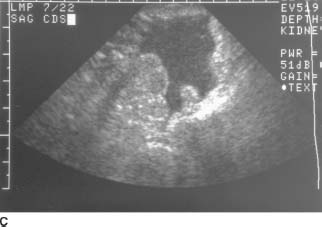
 FIGURE 9-9C Sagittal endovaginal sonogram of the pelvic cul-de-sac. Echogenic free fluid surrounds loops of bowel in the cul-de-sac, due to hemoperitoneum.
FIGURE 9-9C Sagittal endovaginal sonogram of the pelvic cul-de-sac. Echogenic free fluid surrounds loops of bowel in the cul-de-sac, due to hemoperitoneum.
 The differential diagnosis includes conditions found in patients with a positive serum β-HCG but no ultrasound evidence of an intrauterine pregnancy:
The differential diagnosis includes conditions found in patients with a positive serum β-HCG but no ultrasound evidence of an intrauterine pregnancy:
 Early intrauterine pregnancy: This is unlikely because there are abnormal findings in both the adnexa and the cul-de-sac.
Early intrauterine pregnancy: This is unlikely because there are abnormal findings in both the adnexa and the cul-de-sac.
 Spontaneous abortion: This condition is also unlikely because of the abnormalities in the adnexa and the cul-de-sac.
Spontaneous abortion: This condition is also unlikely because of the abnormalities in the adnexa and the cul-de-sac.
 Gestational trophoblastic disease: This disease is unlikely because the endometrial stripe is normal. There is no evidence of an echogenic intrauterine mass with multiple internal cysts, the finding usually seen in patients with gestational trophoblastic disease.
Gestational trophoblastic disease: This disease is unlikely because the endometrial stripe is normal. There is no evidence of an echogenic intrauterine mass with multiple internal cysts, the finding usually seen in patients with gestational trophoblastic disease.
 Ectopic pregnancy: This is the best diagnosis because demonstration of an “adnexal ring sign”in conjunction with sonographically demonstrable hemoperitoneum is highly suggestive of ectopic pregnancy in a patient with a positive β-HCG and no intrauterine pregnancy.
Ectopic pregnancy: This is the best diagnosis because demonstration of an “adnexal ring sign”in conjunction with sonographically demonstrable hemoperitoneum is highly suggestive of ectopic pregnancy in a patient with a positive β-HCG and no intrauterine pregnancy.
DIAGNOSIS
Left ectopic pregnancy
KEY FACTS
Clinical
 The spectrum of clinical symptoms ranges from pelvic pain and vaginal bleeding (often clinically indistinguishable from spontaneous abortion) to catastrophic intra-abdominal hemorrhage.
The spectrum of clinical symptoms ranges from pelvic pain and vaginal bleeding (often clinically indistinguishable from spontaneous abortion) to catastrophic intra-abdominal hemorrhage.
 A β-HCG is necessary to interpret the ultrasound findings: a negative serum β-HCG effectively excludes an ectopic pregnancy.
A β-HCG is necessary to interpret the ultrasound findings: a negative serum β-HCG effectively excludes an ectopic pregnancy.
 The “classic clinical triad”suggesting ectopic pregnancy is amenorrhea, pain, and palpable adnexal mass. This triad, however, is often not present.
The “classic clinical triad”suggesting ectopic pregnancy is amenorrhea, pain, and palpable adnexal mass. This triad, however, is often not present.
 All women with positive β-HCG should be considered at risk for ectopic pregnancy. The following groups are at especially high risk: history of PID, intrauterine contraceptive device, prior ectopic pregnancy, tubal reconstructive surgery, prior tubal ligation, or invitro fertilization.
All women with positive β-HCG should be considered at risk for ectopic pregnancy. The following groups are at especially high risk: history of PID, intrauterine contraceptive device, prior ectopic pregnancy, tubal reconstructive surgery, prior tubal ligation, or invitro fertilization.
 A minority of patients with ectopic pregnancy are critically ill and hemodynamically unstable due to massive intra-abdominal hemorrhage. They require rapid fluid resuscitation and immediate laparotomy, and there may be no time for ultrasound imaging in this group.
A minority of patients with ectopic pregnancy are critically ill and hemodynamically unstable due to massive intra-abdominal hemorrhage. They require rapid fluid resuscitation and immediate laparotomy, and there may be no time for ultrasound imaging in this group.
Radiologic
 Ultrasound is the imaging procedure of choice in the patient with a suspected ectopic pregnancy.
Ultrasound is the imaging procedure of choice in the patient with a suspected ectopic pregnancy.
 One of the main goals of ultrasound is to assess whether there is an intrauterine pregnancy. An intrauterine pregnancy and a coexistent ectopic pregnancy is a rare entity, so if an intrauterine pregnancy is documented, it is generally considered safe to assume there is no ectopic pregnancy.
One of the main goals of ultrasound is to assess whether there is an intrauterine pregnancy. An intrauterine pregnancy and a coexistent ectopic pregnancy is a rare entity, so if an intrauterine pregnancy is documented, it is generally considered safe to assume there is no ectopic pregnancy.
 An intrauterine pregnancy can be diagnosed based on the demonstration of at least one of the following ultrasound findings: (1) intrauterine embryo with cardiac activity, (2) intrauterine yolk sac, and (3) intrauterine gestational sac. An intrauterine gestational sac must be distinguished from a pseudogestational sac of an ectopic pregnancy because ectopic pregnancies can frequently be associated with fluid collections within the uterus. Demonstration of either a “double decidual sac sign”or an “intradecidual sign,”in which the uterine cavity is shown to be separate from the developing gestational sac, indicates the presence of an intrauterine gestational sac.
An intrauterine pregnancy can be diagnosed based on the demonstration of at least one of the following ultrasound findings: (1) intrauterine embryo with cardiac activity, (2) intrauterine yolk sac, and (3) intrauterine gestational sac. An intrauterine gestational sac must be distinguished from a pseudogestational sac of an ectopic pregnancy because ectopic pregnancies can frequently be associated with fluid collections within the uterus. Demonstration of either a “double decidual sac sign”or an “intradecidual sign,”in which the uterine cavity is shown to be separate from the developing gestational sac, indicates the presence of an intrauterine gestational sac.
 The only ultrasound finding 100% diagnostic of ectopic pregnancy is identification of an extrauterine embryo with cardiac activity.
The only ultrasound finding 100% diagnostic of ectopic pregnancy is identification of an extrauterine embryo with cardiac activity.
 The adnexal ring sign, produced when echogenic trophoblastic tissue grows on the inner surface of the fallopian tube around the developing gestational sac, is the next most reliable sign of ectopic pregnancy. Other adnexal findings include pelvic and tubal hematomas. These can assume a wide variety of ultrasound appearances depending on the stage of the hematoma.
The adnexal ring sign, produced when echogenic trophoblastic tissue grows on the inner surface of the fallopian tube around the developing gestational sac, is the next most reliable sign of ectopic pregnancy. Other adnexal findings include pelvic and tubal hematomas. These can assume a wide variety of ultrasound appearances depending on the stage of the hematoma.
 In the appropriate setting, fluid in the cul-de-sac should also raise the level of concern for ectopic pregnancy, particularly if it contains low-level echoes suggesting the presence of blood.
In the appropriate setting, fluid in the cul-de-sac should also raise the level of concern for ectopic pregnancy, particularly if it contains low-level echoes suggesting the presence of blood.
 A negative pelvic ultrasound (empty uterus, normal-appearing ovaries, no adnexal masses, and no free fluid in the cul-de-sac) does not exclude an ectopic pregnancy.
A negative pelvic ultrasound (empty uterus, normal-appearing ovaries, no adnexal masses, and no free fluid in the cul-de-sac) does not exclude an ectopic pregnancy.
SUGGESTED READING
Bhatt S, Ghazale H, Dogra VS. Sonographic evaluation of ectopic pregnancy. Radiol Clin North Am 2007;45:549–560.
Filly RA. Ectopic pregnancy: The role of sonography. Radiology 1987;162:661–668.
Hertzberg BS. Ultrasound evaluation for ectopic pregnancy. Radiologist 1994;1:11–18.
Lin EP, Bhatt S, Dogra VS. Diagnostic clues to ectopic pregnancy. Radiographics 2008;28:1661–1671.
Nyberg DA, Hughes MP, Mack LA, Wang KY. Extrauterine findings of ectopic pregnancy at transvaginal US: Importance of echogenic fluid. Radiology 1991;178:823–826.
Parvey RH, Maklad N. Pitfalls in the transvaginal sonographic diagnosis of ectopic pregnancy. J Ultrasound Med 1993;3:139–144.
BARBARA S.
HERTZBERG
HISTORY
A 38-year-old pregnant woman presents with third-trimester bleeding.
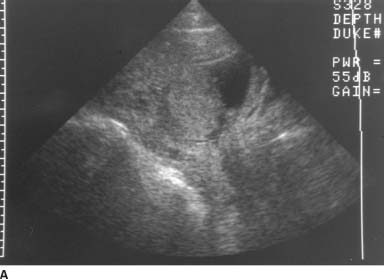
VFIGURE 9-10A Midline sagittal transabdominal sonogram of the lower uterus and cervix. There is a posterior placenta. The lower edge of the placenta overlies the endocervical canal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


