Introduction
Noninvasive imaging during and following carotid artery and peripheral arterial interventions can improve procedural and patient outcomes. Duplex ultrasound imaging alone or in conjunction with extremity systolic blood pressure measurements provides accurate diagnostic information sufficient to identify technical problems and occlusive lesions that can occur following open surgical procedures and endovascular therapy. Color and pulsed Doppler imaging can be performed during interventions with the goal of improving the technical precision of carotid and extremity arterial procedures. Abnormalities at the repair site (stenosis, kinks, dissection, thrombus, low-volume flow) interfere with normal functional patency and contribute to early failure. Routine duplex testing has shown that residual stenosis is a common finding following percutaneous transluminal angioplasty (PTA; 5% to 25%) as well as infrainguinal vein bypass (10% to 15%). The incidence of arterial repair failure is low, 2% to 7% at 1 and 3 years, respectively, when duplex-detected lesions are corrected.
In outpatients, duplex testing is the cornerstone of a vascular laboratory-based surveillance program. Serial testing is performed initially to confirm that the repair simulates normal arterial blood flow and thereafter to detect developing stenosis caused by fibrointimal (myointimal) hyperplasia, a common mode of arterial repair failure, and to identify atherosclerotic disease progression. Repeat intervention based on duplex velocity criteria has been shown to increase long-term patency compared with a clinical follow-up regimen that relies on a recurrence of symptoms or signs of peripheral arterial occlusive disease.
Intraprocedural Duplex Ultrasound Assessment
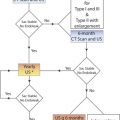
Assessment of an arterial intervention by inspection, pulse palpation, and continuous-wave handheld Doppler signal analysis is safe and easy to perform but lacks diagnostic sensitivity for moderate to severe stenosis or intraluminal defects (dissection, intimal flaps and thrombus). Although fluoroscopic digital subtraction angiography (DSA) is considered a “gold standard” for arterial repair assessment, the technique is invasive, requires arterial puncture, entails radiation exposure, and has the risk of contrast-induced nephrotoxicity. Interpretation of DSA studies, even with multiple imaging runs, is prone to false-negative errors. Duplex testing has emerged as a preferred intraprocedural diagnostic technique because it provides real-time high-resolution imaging coupled with the physiologic information of Doppler waveform analysis. Essentially all open and endovascular vascular procedures can be evaluated with an appropriately selected transducer (either placed directly over the repair or transcutaneous) using a set of interpretative criteria ( Table 16.1 ). Endovascular interventions of the extracranial carotid, subclavian, renal, or mesenteric arteries should be assessed using either DSA alone, with catheter pullback systolic pressure measurement confirming a gradient of less than 10 mm Hg across the repair, or with intravascular ultrasound in selected cases. For open arterial surgical repairs, (carotid endarterectomy [CEA], renal/visceral artery bypass or endarterectomy, extremity arterial bypass), intraoperative duplex ultrasound imaging and the application of Doppler velocity criteria selected to have high diagnostic accuracy (sensitivity and negative predictive value rates of >90%) should be considered. Procedural duplex imaging can be completed within 5 to 10 minutes and is more convenient than completion arteriography.
| Procedure | Angiogram a to Measure Degree of Stenosis | Measurement of Systolic Arterial Pressure Gradient b | Duplex Ultrasound c |
|---|---|---|---|
| Open surgical repair | |||
| Carotid endarterectomy | Yes, <20% | Not used | Yes, PSV <150 cm/s |
| Renal bypass | Not used | Not used | Yes, PSV <180 cm/s |
| Mesenteric bypass | Not used | Not used | Yes, PSV <180 cm/s |
| Infrainguinal bypass | Yes, <30% | Not used | Yes, PSV <180 cm/s |
| Endovascular intervention-percutaneous transluminal angioplasty | |||
| Carotid stent-angioplasty | Yes, <20% | Not used | Yes, PSV <150 cm/s |
| Renal PTA | Yes, <30% | Yes, <10 mm Hg | Not used |
| Mesenteric PTA | Yes, <30% | Yes, <10 mm Hg | Not used |
| Iliac PTA | Yes, <30% | Yes, <10 mm Hg | Yes, PSV <180 cm/s |
| Infrainguinal PTA | Yes, <30% | Not used | Yes, PSV <180 cm/s |
| Vein bypass balloon PTA | Yes, <30% | Not used | Yes, PSV <180 cm/s |
a Criteria for acceptable residual stenosis, expressed as diameter reduction.
b Catheter measurement of pressure gradient during systole across angioplasty site.
c Peak systolic velocity threshold for revision of residual stenosis, used in conjunction with B-mode imaging criteria of stenosis and peak systolic velocity ratio of >2.0 at site of abnormality.
The type of abnormality seen during imaging varies with the procedure type, but a stenosis caused by residual disease, plaque dissection, or improper suturing is the most common abnormality seen with either duplex scanning or DSA. When color flow imaging and the Doppler waveform indicate the presence of a severe stenosis, the stenosis probably reduces volume blood flow and downstream pressure. In severe cases, this causes stasis proximal (upstream) to the stenosis and can promote platelet-thrombus formation and thrombosis. Failure to identify the abnormal repair increases the likelihood of procedure failure, an outcome that requires a secondary salvage procedure and is associated with increased morbidity and health care costs.
It is recommended that a vascular technologist participate in duplex ultrasound evaluations performed in the operating room or angiography suite. The technologist assists in instrument setup to optimize image resolution and color Doppler settings that are essential for high-quality imaging. For intraoperative assessment, a 10- to 15-MHz linear array transducer with a small footprint affords high-resolution imaging and can be placed within the surgical wound, directly on the repair site. For transcutaneous imaging of bypass grafts or extremity angioplasty/atherectomy procedures, 5 MHz or higher frequency linear array transducers are necessary to image the deeper-positioned vessels. Acoustic coupling necessary for duplex imaging is achieved by placing the transducer within a sterile plastic sleeve containing acoustic gel. Acoustic coupling to the patient is achieved with sterile gel on the prepped operative field or with saline in the wound.
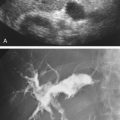
Reluctance to adopt routine intraprocedural duplex assessment is based on the false belief that testing is difficult to perform and to interpret, whereas clinical assessment and arteriography provide equivalent diagnostic accuracy. Intraoperative duplex assessment of infrainguinal vein bypass doubles the immediate revision rate compared with completion DSA, and early (30-day) thrombosis/revision rate is halved. The recommended velocity criteria thresholds for repair of a duplex-detected stenosis in a vein bypass graft vary. However, a peak systolic velocity (PSV) greater than 150 to 180 cm/s associated with a velocity ratio (Vr) greater than 2 across the imaged stenosis ( Fig. 16.1 ) has been shown to predict both lower extremity bypass and angioplasty site failure. In a blinded prospective trial, a Vr of 3 or above achieved a high predictive value for occlusion or future graft revision with a sensitivity of 71%, a value lower than that for a Vr of 2 or more. Revision rates based on duplex testing are higher with infrainguinal bypass to below the popliteal artery (17%), when arm (27%) versus saphenous (15%) vein conduit is used, and when percutaneous atherectomy is performed (20%) versus balloon (15%) or stent angioplasty (5%).
- •
Intraprocedural imaging can be performed after placement of the transducer in a gel containing sterile bag and ensuring acoustic coupling with:
- •
sterile gel if on top of the sterile field
- •
sterile saline in the operative wound
- •
- •
A small footprint transducer with high-frequency (10 to 15 MHz) range can be placed in a wound.
- •
A high-frequency transducer (5 MHz or more) can be used via a transcutaneous approach or placed on a graft conduit.
- •
A peak systolic velocity ratio of 2 or more and a peak systolic velocity above 150 to 180 cm/s are very sensitive indices of a residual stenosis.

Surveillance After Carotid Intervention
On the basis of prospective randomized clinical trials such as the Asymptomatic Carotid Atherosclerosis Study (ACAS) and North American Symptomatic Carotid Endarterectomy Trial (NASCET), carotid artery endarterectomy has been shown to be more effective than medical therapy in the prevention of stroke. Carotid artery stent placement has also been shown to be equivalent to CEA in the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST). The efficacy of CEA and carotid artery stenting (CAS) is highly dependent on completing the procedure with a low perioperative morbidity rate (<5%) and producing a durable repair with a low incidence of recurrent disease or occlusion.
Studies have documented that the rate of recurrent carotid artery stenosis following CEA is relatively low especially if an early imaging study does not indicate significant abnormality. However, monitoring the progression of internal carotid artery (ICA) stenosis with ultrasound on the nonoperated side may justify its use. When intraoperative or early duplex imaging of a CEA procedure shows the absence of a significant blood flow abnormality, the rate of restenosis is very low. However, the presence of a peak systolic velocity elevation of more than 150 cm/s or the presence of a luminal lesion of 2 mm or more should be considered indications for surgical revision.
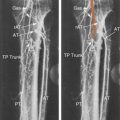
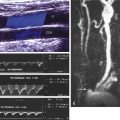
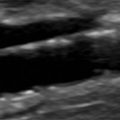
B-mode ultrasound images following CEA will often show characteristic features. After endarterectomy, the normal intima-media stripe is absent at the beginning of the endarterectomy and often causes a step or shelf at the beginning of the repair site ( Fig. 16.2 ). The arteriotomy closure sutures can appear as bright reflectors in the anterior wall typically starting in the distal common carotid artery (see Fig. 16.2 ), but this finding may not be seen in all patients. As wall remodeling occurs over the subsequent weeks to months, wall thickening develops due to fibrointimal hyperplasia and a neointima develops ( Fig. 16.3 ). The clinical importance of the neointima is normally minor unless it causes severe lumen reduction and an increase in PSV ( Fig. 16.4 ). The development of a neointima is easily seen following a patch closure of the arteriotomy site (see Figs. 16.3 and 16.4 ). However, primary patch placement will prevent recurrent stenosis in many cases.




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree