Introduction
Endovascular aneurysm repair (EVAR) uses endografts, also called endovascular grafts, covered stent-grafts, or transluminally placed endovascular grafts to isolate an aortic aneurysm from the circulation. EVAR is preferred to open abdominal aortic aneurysm (AAA) repair. Patients undergoing EVAR will probably require routine, lifelong follow-up, and imaging surveillance.
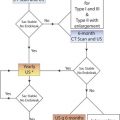
Frequent assessment and objective follow-up are critical following EVAR. Color Doppler ultrasound has been used for aortic endovascular graft evaluation and has the advantage of being noninvasive, inexpensive, rapid, safe, nontoxic, easily repeatable, and well tolerated by patients. This technique has already become an important tool in both the planning and the postoperative evaluation of endovascular grafts placed for a variety of vascular lesions and complications. Color Doppler imaging combines many of the ideal features of both angiography and computed tomography (CT). It allows the examiner to make both quantitative and qualitative assessments of blood flow through the endovascular graft. The combination of color Doppler imaging and Doppler waveform analysis can easily differentiate normal blood flow patterns from abnormal patterns associated with various pathologies. Because color Doppler is relatively inexpensive, easily repeatable, and without known risks, it has played an important role in the postprocedure surveillance follow-up of endovascular interventions (see Chapter 16 ).
The primary objectives of the color Doppler examination following endovascular AAA repair will be:
- •
to assess blood flow patterns through the main graft conduit and limbs, including the identification of any kinking, stenosis, or thrombosis
- •
to measure maximal residual aneurysm sac diameter
- •
to determine whether there is any blood flow in the aneurysm sac (endoleak)
- •
to characterize the type of endoleak, if present
Overview of EVAR
General principles
As discussed in Chapter 24 , intervention on the enlarging AAA is undertaken to reduce, if not eliminate, the risk of catastrophic aneurysm rupture.
Endografts are a combination of metallic stents and affixed prosthetic graft materials. The stent portion gives radial strength to the stent-graft, and the graft material covers it and makes it impermeable. In essence, a perfectly anchored stent-graft only permits blood to flow through its conduit. The stent portion also stabilizes the position of the stent-graft by anchoring it to healthy portions of the aorta and iliac arteries. Instead of using sutures to stabilize the position of the graft and its limbs, the net radial force exerted by the metallic components of the stent pushes outward against the aortic and arterial segments selected for placement. The stent can be made of nitinol, stainless steel, or a cobalt-chromium alloy. The fabrics typically used as the prosthetic graft material component of the endograft include Gore-Tex (PTFE) and Dacron.
Once the endograft is fixed into position above and below an aneurysm, blood should only flow through the graft. The endograft effectively excludes the aneurysm sac from the effects of blood pressure and blood flow ( Fig. 25.1 ). Endovascular grafts come in many types and configurations ( Fig. 25.2 ).


The endovascular placement of a stent-graft is currently favored over open surgical intervention because it reduces the relatively high morbidity and mortality associated with traditional open operative repair. The first series of endovascular placed stent-grafts for the repair of AAAs in high-risk patients was reported in 1991. Since then, significant advances in the design of endografts have facilitated their deployment for the treatment of aortic and aortoiliac aneurysms.
Although many devices have been granted US Food and Drug Administration (FDA) approval for the treatment of aortoiliac aneurysms and are available for widespread use in the USA, the list keeps growing with various fenestrated models now available for suprarenal implantation.
Because most aortic aneurysms are infrarenal, the typical stent graft is deployed in an infrarenal location. The proximal anchor point is in the infrarenal aorta immediately below the lowest renal artery and the inferior anchor points are in both common iliac arteries ( Fig. 25.3 ). It is not uncommon for endovascular aortic aneurysm repair to be supplemented by other ancillary procedures such as graft extension, femorofemoral artery bypass, intra-arterial coil vessel occlusion, or other vessel occlusion procedures ( Fig. 25.4 ). The device has an uncovered metal component when there is need for suprarenal fixation. The uncovered proximal metal component of the stent can cross the orifices of the renal arteries. This design is thought to provide better fixation of the graft to the surrounding aortic wall, thereby reducing the potential for graft migration and providing a better proximal seal. The uncovered component also allows perfusion of the kidneys. Newer endovascular grafts can treat a variety of complex arterial pathologies, and their surveillance becomes more complex. Examples include fenestrated endografts that allow coverage of the renal arteries as well as the celiac axis and superior mesenteric arteries if needed.


Although the endovascular repair of AAAs offers many benefits ( Table 25.1 ), there are several potential complications specific to this technique. The most significant of these complications are endoleaks and, in early series, graft migration. Although endoleaks are still common, endograft migration, deformation, or occlusion are uncommon because of new designs and broader experience with the technique.
| Procedure is performed from a remote site and avoids laparotomy |
| Small incisions (femoral, brachial, or rarely carotid artery cut-down for access) |
| No prolonged aortic clamping and potential for spinal cord ischemia |
| Decreased length of stay or no need for stay in intensive care unit |
| Decreased length of hospitalization (1–2 days for endovascular vs. 6–8 days for open repair) |
| Decreased recovery time (to resumption of normal activity level) |
Endograft placement is performed to reduce pressure in the aneurysm sac and therefore prevent aneurysm rupture. An endoleak is an undesired source of pressure within the aneurysm sac and is defined as blood flow outside the endovascular graft but within the aortic aneurysm sac. These leaks might increase intrasac pressures and make it more likely for the aneurysm sac to rupture because the pressure can cause aneurysm enlargement, and the presence of blood flow makes it likely to have active hemorrhage when the aneurysm sac ruptures. The presence of an endoleak therefore negates the primary goal of the endovascular procedure and indicates that the aneurysm remains inadequately treated. Considerable progress in patient selection and endovascular technique has reduced the overall rate of all types of complications seen after EVAR ( Table 25.2 ). The optimal method of follow-up following endograft placement is contrast-enhanced CT with delayed imaging. However, this method is relatively invasive because of the need of intravenous access, is expensive, involves radiation exposure to the patient, and has increased risk for possible contrast nephrotoxicity. Color Doppler ultrasound and contrast-enhanced ultrasound are increasingly and successfully being used to follow patients following endograft placement. Patients who may require further intervention can be readily identified.
| Aneurysm growth |
| Embolization |
| Endoleak a |
| Fabric tears |
| Graft infection |
| Graft migration a |
| Limb thrombosis |
| Limb separation |
| Stent and/or attachment site fracture |
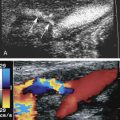
It is important to try to determine the origin of any blood flow signals identified within the aneurysm sac ( Table 25.3 ) because the source of the blood flow signals (endoleak) and associated characteristics will help determine subsequent treatment options.
| Type of Endoleak | Description | Outcome |
|---|---|---|
| 1a, 1b | Endoleak whose origin is at the proximal (1a) or distal (1b) endograft attachment site. | Needs intervention |
| 2 | Endoleak originating from a branch vessel. Possible sources include patent lumbar (posterior aspect of the aorta), inferior mesenteric (anterolateral to the aorta), accessory renal or hypogastric (internal iliac) arteries, or other patent branches of the abdominal aorta such as the right gonadal artery. These are best seen on transverse imaging. | Significance unknown. Monitoring of aortic aneurysm sac size needed. |
| 3 | Endoleak that originates at the junctions between components of modular devices or from fabric tears within the graft. | Needs intervention |
| 4 | Blood flow through the endograft or filling of the aneurysm sac because of endograft porosity. | Usually a transient phenomenon that is self-limited and resolves spontaneously once blood clots in the aneurysm sac. |
| 5 | Unknown origin or endotension | Close monitoring needed. If aneurysm size continues to increase, consider reinforcing the inside of the endograft or performing an open procedure. |
Cross-sectional diameter measurements are recorded at each visit to determine maximum aneurysm size. When an aneurysm sac is excluded from the circulation, it should remain stable or decrease in size over time. Any increase in size suggests that there is preserved blood flow into the aneurysm sac (endoleak) with associated increase in blood pressure, which is a continued risk for rupture. However, increases in size have been reported without CT, angiographic, or color Doppler evidence of endoleak (endotension). Endotension is defined as persistently elevated pressures within the aneurysm sac, without a known source, and can be documented by direct pressure measurements made within the sac.
It is also important to determine whether the distal arterial circulation has been preserved by ensuring that there are no kinks or obstructions within the body of the endovascular graft, the graft limb(s), and the inflow and outflow arteries. This can be done using a well-defined protocol. When abnormalities are detected by color Doppler imaging, contrast arteriography and CT may be used to characterize the abnormality further. The following section describes a protocol for assessing endovascular grafts performed for the repair of isolated aneurysms of the abdominal aorta as well as aortoiliac aneurysms.
- •
Endovascular repair is now the preferred approach to the treatment of AAAs.
- •
Once an endograft is placed in an aneurysm:
- •
the excluded portion is called the aneurysm sac
- •
the aneurysm sac is isolated from the effects of the circulation and blood flow, but more importantly from the effects of pressure
- •
- •
Proper placement of the endograft is required to seal off the aneurysm sac from the effects of the circulation.
- •
There are many types of endografts, each adapted to specific anatomy of the aneurysm, the native aorta, and the iliac arteries.
- •
Endoleaks occur at attachment sites, within the endograft, or through native artery branches.
- •
Following successful endograft placement, the aneurysm sac diameter should stabilize or decrease.
Ultrasound Examination
An endograft evaluation can be as short as 30 minutes or up to 2 hours depending on the complexity of the intervention and the patient’s body habitus. This allows enough time to prepare the patient and room, to perform the imaging, and to provide a preliminary report for the interpreting physician.
Patient preparation
As with all abdominal scanning, the quality of the examination may be degraded in patients with a large body mass index or when abundant bowel gas is present. Patient preparation may be necessary. The patient should fast overnight or for at least 8 hours before the study. This will decrease the amount of intestinal gas and facilitate visualization of the graft and attachment sites. Usually, no other patient preparation is necessary.
Technologist preparation
The technologist/sonographer can take a brief history of symptoms of claudication (hip, buttock, or lower extremity pain on exertion) and impotence (as applicable) before the ultrasound evaluation. Although a physical examination is typically not performed, some laboratories include palpation of the aortic and femoral pulses. An ankle-brachial index and/or pulse volume recordings can be obtained bilaterally and compared with the preoperative measurement, where available, to ensure that baseline blood flow to the extremities has been maintained.
To perform a thorough and optimal examination of the endovascular graft, the examiner should be familiar with the various endovascular graft designs. Optimally, the examiner should have information about the endograft configuration including the locations of the proximal and distal attachment sites. This information is used to document endovascular graft migration (if it occurs) and to identify potential endoleak sites, especially the proximal and distal attachment points for type 1 endoleaks ( Fig. 25.5 ) or for type 3 endoleaks (at joining graft segments or because of a defect in the graft material ( Fig. 25.6 ). In addition, review of the operative report or discussion with the interventionalist or surgeon is recommended to determine whether the following apply: (1) coil embolization of branch vessels or use of other vessel occlusion devices, (2) supplemental proximal or distal arterial reconstructive procedures, and (3) other segments that have been treated with stents, either within the endovascular graft to support a portion of the graft or in the native artery to treat occlusive disease (see Fig. 25.4 ). The sonographic examination must include the iliac stent graft components and related arterial occlusion and reconstruction procedures.


Ultrasound equipment
The examination is performed using a high-resolution, state-of-the-art, duplex scanner with color flow capability. A low-frequency (2 to 5 MHz) sector or curved array transducer is necessary to visualize these deep structures. A variety of different probes and/or higher-frequency transducers may be needed to accomplish a complete study. The equipment selected must allow enough penetration to permit adequate imaging of the deep structures in the abdomen and pelvis with sufficient color flow sensitivity permitting the detection of slow velocity signals resulting from an endoleak.
Color Doppler imaging is essential to the examination because it allows for quick identification and evaluation of blood flow signals within the endovascular graft and aneurysm sac. The velocity scale (or pulse repetition frequency; PRF) must be adjusted to allow the detection of low-velocity signals in the deep abdomen and pelvis with a narrow color box that will permit higher frame rates. The color Doppler gain needs to be adjusted in order to minimize false-positive signals caused by overgaining and to avoid a false-positive result of endoleak based on the presence of color Doppler signals within the aneurysm sac. A low color Doppler gain may be insufficient to detect the low-amplitude signals of a small endoleak and give a false-negative result. The color flow image is therefore adjusted by overgaining the color sensitivity and then progressively reducing the gain until color noise and color speckling is reduced within the color box.
All images are digitally stored with appropriate digital loops or kept as a combination of images and videotape if an appropriate digital archiving system is not available. This facilitates review by the interpreting physician and review by the sonographer/technologist before follow-up studies.
General considerations
Patients are generally scanned in the supine position initially using a midline approach; however, a left lateral decubitus position (the patient lying on their left side ) facilitates visualization of the entirety of the aneurysm sac. Occasionally, a right lateral decubitus approach may be useful. Technically challenging studies will require multiple views to detect abnormalities that may not be appreciated in any one projection. Any examination that does not achieve visualization of the entire aneurysm sac may be considered of limited diagnostic value, and the examination may need to be repeated as deemed necessary by the interpreting physician.
Technical protocol
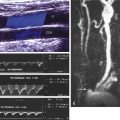
Ultrasound assessment begins with identification of the intra-aneurysm sac portion of the endovascular graft in a transverse plane ( Fig. 25.7 ). The endograft is then followed proximally to the superior attachment site, where the endovascular graft–artery interface is seen. This site is typically at, or immediately distal to, the level of the renal arteries, which are visible in the transverse plane. The renal arteries are important landmarks in the evaluation of endovascular AAA repair.

When the superior end of the endovascular graft is identified, the transducer is turned longitudinally parallel to the long axis of the aorta. Alternatively, longitudinal assessment of the aorta begins immediately inferior to the xiphoid process at the midline or slightly to the left of the midline. After identification of the origins of the celiac or superior mesenteric arteries ( Fig. 25.8 ), scanning distally permits visualization of the stent or fixation component of the endovascular graft, which may cross the orifices of both renal arteries or may be deployed immediately below the takeoff of the lowest renal artery ( Fig. 25.9 ).


The endovascular graft proximal component should be in complete apposition to the aortic wall. The aortic diameter at this site (proximal neck of the aneurysm) is measured and compared on follow-up examinations to assess for dilatation of the aneurysm neck, as this can result in endovascular graft migration and/or type 1 endoleak (see Fig. 25.9 ). Occasionally, the endovascular graft takes a sharp turn immediately distal to the proximal attachment site. This will occur when the aortic aneurysm causes the aorta to have a very tortuous course.
After the proximal fixation site is identified and evaluated, the transducer is moved upward and the suprarenal aorta is visualized and examined for defects, such as dissection and intimal flaps that may have resulted from the introduction and manipulation of devices used during endovascular graft deployment (e.g., catheters, guide wires, sheaths). Blood flow velocity is recorded immediately proximal to the endograft. The body of the endovascular graft is then scanned along its entire length in the color Doppler mode, and a search is made for sites of color flow signals and any filling defects. Doppler waveforms and blood flow velocity estimates are recorded in the intra-aneurysm portion of the graft with particular attention to any sites of kinking and/or twisting that may result in stenosis and decreased blood flow. The iliac limbs of the endograft will often seem to rotate by 180 degrees. This is often done during graft insertion to facilitate connecting the iliac limb to the main graft component (see Fig. 25.3 ).
For aortoiliac or aortofemoral endovascular grafts, the iliac and femoral segments of the endovascular graft (and the femorofemoral crossover graft when present) are then examined for any abnormalities. Doppler waveforms and blood flow velocity estimates are obtained at the distal endovascular graft termination and in the outflow artery distal to the graft. For these and all other velocity measurements, typical procedures are followed: the Doppler angle is maintained at 60 degrees or less, and the angle cursor is aligned so that it is parallel to the vessel/graft wall. The distal end of the endovascular graft is usually attached to the surrounding artery wall by the supporting stent of the endovascular graft but otherwise may be sutured endoluminally in the case of a nonsupported endovascular graft.
To this point, the ultrasound examination has focused principally on the endovascular graft. The focus is then turned to the surrounding aneurysm sac. Special attention is directed to detecting any flow signals outside the endovascular graft (endoleak), to measuring the size of the aneurysm sac (outer wall to outer wall of the original aneurysm), and to assessing for the presence and evolution of clot formation within the aneurysm sac. Gray-scale images are used to look for areas with low echoes within the aneurysm sac because this often represents areas with fresh thrombus or a local blood pool ( Fig. 25.10 ).