Fig. 13.1
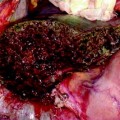
CT scan showing two large tumors in the right lobe of the liver
(c)
superficial lesions adjacent to visceral structures which can be displaced by laparoscopic maneuvers: colon appears to be at greater risk (Fig. 13.2a) than the stomach (Fig. 13.2b) or small bowel for thermally mediated perforation;
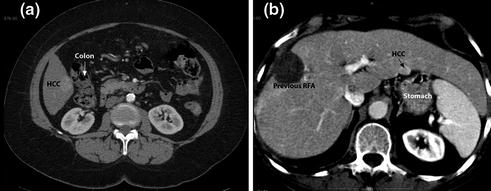

Fig. 13.2
CT scan showing superficial lesions adjacent to visceral structures: colon (a) or stomach (b)
(d)
lesions close to intrahepatic structures (Fig. 13.3): the laparoscopic approach guarantees a lower risk of biliary thermal injuries either performing a “cooling technique” for these lesions or associating a cholecystectomy for lesions close to the gallbladder (Fig. 13.4);
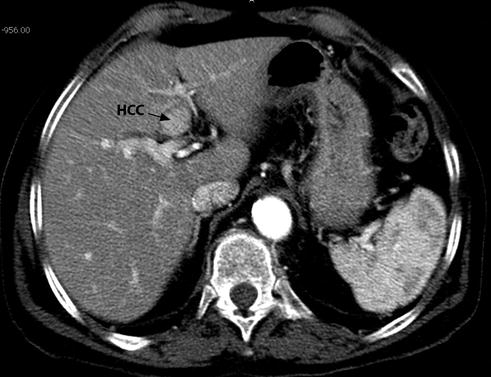
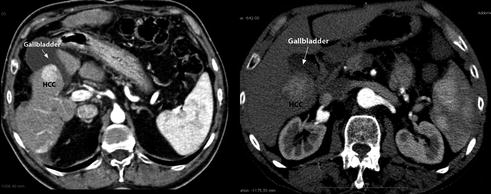

Fig. 13.3
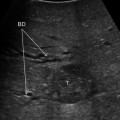
CT scan showing a HCC nodule contiguous to intrahepatic biliary convergence

Fig. 13.4
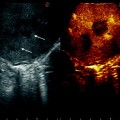
CT scans showing HCC nodules contiguous to gallbladder
(e)
deep-sited lesions with a very difficult or impossible percutaneous approach (Fig. 13.5);
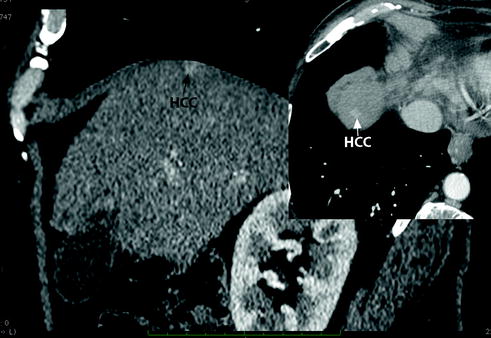

Fig. 13.5
CT scan showing a deep HCC nodule of the hepatic dome not visible upon percutaneous ultrasound examination
(f)
short-term recurrence of HCC following liver surgery, ethanol injection or TACE.
Table 13.1 shows the different reasons to propose an LRFA rejecting a percutaneous approach or a hepatic resection.
Table 13.1
Reasons to reject percutaneous RFA or hepatic resection (HR) for 379 patients submitted to LRFA (more than 1 reason for each patient)
Percutaneous RFA | N (%) | Hepatic resection (HR) | N (%) |
|---|---|---|---|
HCC contiguous to visceral structures | 113 (30 %) | HR > 2 segments | 161 (42 %) |
Superficial lesion | 74 (19 %) | Patients > BCLC A2 stage | 226 (60 %) |
Lesion difficult or impossible to percutaneous US visualization | 230 (61 %) | Local recurrence post-TACE | 37 (10 %) |
LUS staging (for suspected other nodule) | 344 (91 %) | Other concomitant severe disease | 95 (25 %) |
Pts at risk of bleeding (plts < 50,000 and/or INR > 1.2) | 154 (41 %) | Patient refusal | 95 (25 %) |
Age > 78 years | 52 (14 %) |
13.2.2 Liver Metastases
Surgical resection represents the gold standard for the management of LM [10]. Unfortunately, only 10–25 % of patients are amenable to liver resection due to the characteristics of either hepatic lesions (location, number, remnant liver volume) or patients (comorbidities, chemotherapic toxicity, performance status) [11]. The rationale for choosing RFA versus surgical resection in patients with LM has been multifactorial. A large percentage of patients were thought to have disease that was technically unresectable. Most of the remaining patients were poor surgical candidates for a combination of reasons, including clinically important medical comorbidities, advanced age, and aggressive disease. In this setting of unresectable patients, RFA could represent an acceptable indication [12]. The number of lesions should not be considered an absolute contraindication to RFA: nevertheless, most centers preferentially treat patients with five or fewer lesions. The target tumor should not exceed 3 cm in longest axis to achieve best rates of complete ablation with most of the currently available devices.
A particular scenario is if a colorectal cancer patient undergoes laparoscopic surgery for primary disease with synchronous LM. LRFA may be safely used concomitantly with colon resection in patients who may have resectable LM, but liver resection in a synchronous manner would be risky or, if the patient has an unresectable LM; in this case, LRFA could represent the first stage in a two-stage treatment (Fig. 13.6).
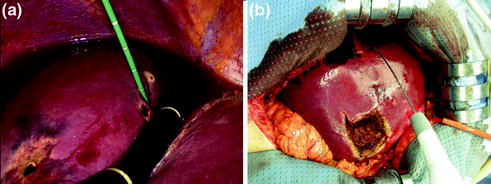

Fig. 13.6
Laparoscopic MWA of two liver metastases in segment 4 during hemicolectomy (a) and in a second time multiple hepatic resections associated to RFA in the right lobe (b)
13.3 Which ‘‘Weapon’’? RFA or MWA
Both radiofrequency and microwave technology are effective. In RFA, high-frequency alternating current causes thermal coagulation and protein denaturation; as the temperature is increased above 45 °C, cellular proteins denature and cell structure is lost. RF ablation can be performed by either monopolar or bipolar techniques; monopolar techniques are more commonly used for tumor ablation [13]. When a monopolar technique is used, a large dispersive electrode (grounding pad) is usually placed on the patient’s thigh. Current passing through tissue from the active electrode leads to ion agitation, which is converted by means of friction into heat. The process of cellular heating induces almost immediate and irrepairable cellular damage, which leads to coagulation necrosis. The nature of the thermal damage caused by RF heating depends on both the tissue temperature achieved and the duration of heating. The diameter of coagulation necrosis was also found to be influenced by electrode diameter [14]. Currently, two RF technologies are commercially marketed (Fig. 13.7): one of these devices consists of a needle with a movable hub that deploys a variable number of curved electrodes into the adjacent tissue in a radial manner. The configurations of the multiple electrodes are designed to produce large spherical thermal injuries. The second device consists of a straight, internally cooled needle electrode. The internal cooling is designed to prevent charring of the adjacent tissues and thus any larger thermal injury. In our experience, for the particular indications in the laparoscopic approach, the “cooled” needle is preferred [4]. The major factor limiting the size of the thermal injury produced by all two devices is hepatic perfusion. Normal blood flow through the liver produces perfusion-mediated tissue cooling, above all if the lesion is near to a larger vessel (heat sink effect) [15].
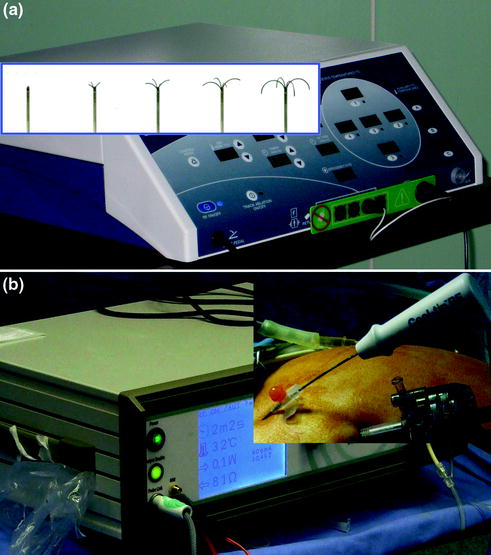

Fig. 13.7
RF technologies: one of these devices consists of a needle with a movable hub that deploys a variable number of curved electrodes (a) and the second device consists of a straight, internally cooled needle electrode (b)
MWA is another local ablation therapy, which has been used for more than 20 years [16]. It has recently attracted considerable attention because of the tremendous progress in microwave technology [17]. Microwaves generate heat by oscillation of dipole water molecules within tissues. Frequencies of 915 MHz and 2.45 GHz are currently used for tissue ablation, with a single, dual, or triple antenna (Fig. 13.8). The advantage of MWA is that the heating is primarily active, and the transmission of microwaves in living tissue is not limited by tissue desiccation and charring. In addition, MWA does not require use of earth plates on the patient’s body, thus avoiding potential earth plate burn injury that can occur with RFA. With a single application of a 2.45 GHz system, it can create a 4 × 6 cm ablation in 4 min and a 5 × 7 cm ablation in 8 min. MWs offer all of the same benefits as RFA energy for thermal ablation but they are not as dependent on tissue properties and have the ability to heat faster in a larger volume. Thus, MWs are less susceptible to perfusion or heat sinks and may be able to penetrate deeper into low-conductivity materials [18]. On the other hand, the MW antenna usually has a larger diameter (14 G) and the lack of heat sink effect increases the risk of vascular and biliary injuries. So, it’s impossible to establish which tool is better: it’s recommended to have the possibility to choose which technology depending on size and location of the tumor.
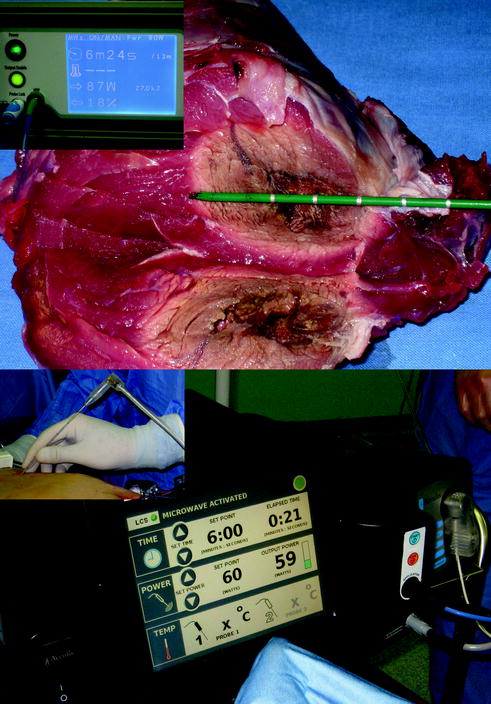

Fig. 13.8
MW technologies
13.4 Thermal Ablation: The Procedure
13.4.1 Operative Room
An efficiently operating room setup for LRFA/MWA is critical for success. A proper table that allows the patient to be placed in both full Trendelemburg and reverse Trendelemburg positions during operation is essential. The optimal situation is to have the ultrasound monitor in the same line of vision as the monitor for the laparoscopic telescope. A convenient way to visualize both laparoscopic and ultrasound images simultaneously is to employ a screen-splitting device for a single monitor: a picture-in-picture visualization is particularly helpful in the LRFA procedure (Fig. 13.9). Generally, the ultrasound unit is located on the right side of the patient adjacent to the armboard with the laparoscopic equipment off the right shoulder of the patient: the surgeon stands at the right side of the patient (Fig. 13.10). Also the RFA/MWA machine is positioned on the patient’s right side. An equipment checklist is necessary to ensure that all items are available:
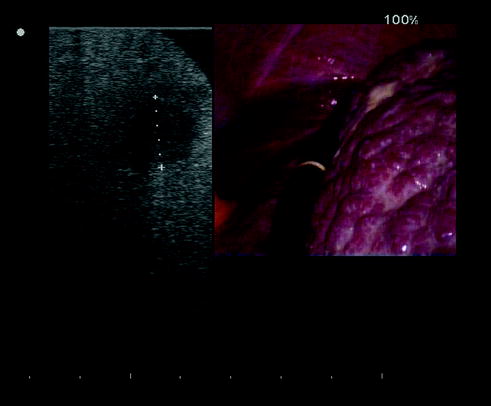
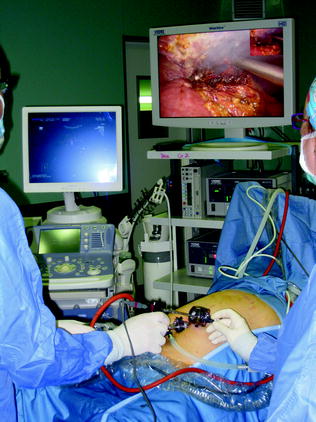

Fig. 13.9
A screen-splitting device for a single monitor in order to visualize both laparoscopic and ultrasound images simultaneously

Fig. 13.10
The ultrasound unit is located on the right side of the patient with the laparoscopic equipment off the right shoulder of the patient
laparoscopic equipment (generally housed in a cart on wheels):
monitor: flat panel monitor has better resolution and it is more mobile than the than traditional monitor;
insufflator: we recommend a high-flow insufflator capable of delivering flow rates up to 30 l/min;
camera–processor unit: the laparoscopic camera can be either single chip or three chip; these provide high-quality color reproduction and highest degree of fidelity;
light source: a high-intensity light source is a prerequisite for a satisfactorily bright laparoscopic image;
laparoscopic optics: a 30 or 45° laparoscope is preferable for closeup viewing of the surface architecture of the liver and for providing guidance to the electrode under direct vision.
In addition, by rotation of the telescope, it allows different angles of inspection during the electrode infission;
energy sources for coagulation and cutting: standard unipolar or bipolar electrocautery can be used for hemostasis and dividing tissue. For cirrhotic patients with extremely vascular tissue such as adhesions, alternative energy sources such as ultrasonic coagulation may be more suitable.
ultrasound equipment: we used an ultrasound machine connected to a laparoscopic ultrasound (LUS) probe with a flexible tip, 10 mm in diameter and 50 cm in length. A 5–7.5 MHz linear-array transducer was side-mounted near the tip of the shaft. The length of the transducer surface was 38 mm, which produced an image footprint of approximately 4 cm in length and 6 cm in depth. Recently, we also used a microconvex probe that permits the application of intravenous ultrasound contrast agents during LUS of the liver: the addition of contrast enhancement during the intraoperative ultrasound will improve image conspicuity, correct diagnosis of new malignant nodules, ablation efficacy in hepatic tumors, and oncologic margin outcomes (Fig. 13.3);
suction irrigator;
table with small retractors for umbilical incision, trocar cannulae (size and number depend on the planned operation), and laparoscopic instruments (atraumatic graspers, bowel grasping forceps, coagulated hook and scissors);
18 G tru-cut biopsy needle: it is useful to use a cutting needle with an automatic trigger mechanism in order to hold the probe with one hand and the needle with the other. Because of the presence of pneumoperitoneum which separates the surface of the liver from the abdominal wall, the use of longer needles (25–27 cm) can be necessary for lesions localized in the posterior segments or in the highest part of the liver (segments 4a and 8);
RFA/MWA machine and electrode/antenna: actually we used a dual ablation system which in the same hardware has both a MWA and an RFA energy generator. For RFA technology, we prefer a 17 G internally cooled electrode with an exposed tip length of 3 cm and shaft length of 250 mm. For MW technology, we use a 14-G interstitial antenna with a shaft length of 270 mm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree