Category
Age (years)
55.12 ± 14.08
Sex (M/F)
20/12
Original/metastasis
9/49
Tumor number (1/2/3)
58
≤3 cm/3–5 cm/>5 cm
35/11/12
Tumor diameter (cm)
3.33 ± 2.26
Tumor location (abdominal wall/chest wall/groin)
42/13/3
Ablation power (W)
45.26 ± 7.58
Ablation needle
1.57 ± 0.6
Ablation session
1.1 ± 0.34
Follow-up (months)
13.28 ± 7.54
Ultrasound-guided MWA was performed for 58 tumors in 32 patients. The follow-up time was 3–26 months (13.28 ± 7.54 months). The observation period was less than 6 months for six patients (6/32, 18.75 %), 6 months to 1 year for nine patients (9/32, 28.13 %), 1–2 years for 13 patients (13/32, 40.63 %), and 2–3 years for four patients (12.5 %). Indications for MWA in these 32 patients were advanced age or poor surgical candidates with significant comorbidities (seven patients), poor liver or renal function (eight patients), other ineffective therapies (five patients), and patient preference (12 patients).
Microwave ablation output power ranged from 30 to 50 W, and an output setting of 50 W was routinely used during ablation sessions, relatively lower than that used in liver lesions. Desired temperatures were attained in no more than 1,180 s (range 120–1,180). Thermocouple was used during ablation for the tumors adjacent to the high-risk locations. In this study, four tumors were adjacent to the gallbladder, three tumors were adjacent to the intestinal tract, and three tumors were adjacent to the pleuroperitoneum. We used one thermocouple to monitor the temperature, and the maximum was 50 °C in these tumors. The mean treatment session was 1.1 per person (1–3).
On the basis of follow-up imaging finding, technique effectiveness was achieved in 54 of 58 (93.1 %) tumors. Four lesions with local tumor progression were discovered at 1, 3, 4, and 15 months after MWA, with the ablation nodule size of 4, 6.2, 7.4, and 7.9 cm, respectively (Table 21.2). Therapeutic options offered to patients with local tumor progression included chemotherapy for one patient and iodine-125 seed implantation for three patients.
Table 21.2
Comparison of ablation results in different tumor sizes
Parameters | ≤3 cm | 3–5 cm | >5 to ≤10 cm | p value |
|---|---|---|---|---|
Tumor number | 35 | 11 | 12 | N/A |
Tumor size (cm) | 1.9 ± 0.6 | 3.8 ± 0.6 | 7.0 ± 1.6 | 0.0001 |
Number of antenna insertions | 1.3 ± 0.5 | 1.8 ± 0.6 | 2 ± 0.6 | 0.11 |
Emission time (s) | 395.1 ± 213.1 | 877.3 ± 409.9 | 1,115.3 ± 345.3 | 0.0001 |
Technique effectiveness (%) | 100 (35/35) | 90.9 (10/11) | 75 (9/12) | 0.01 |
LTP (%) | 0 (0/35) | 9.1 (1/11) | 25 (3/12) | 0.01 |
No major complications occurred during or immediately after MWA. Ten patients experienced grade 1 pain according to the standardization of terms and reporting criteria for image-guided tumor ablation [18] after the treatment, and no analgesic medication was needed. Minimal asymptomatic pleural effusion and ascites was found in three patients, which was resolved spontaneously in 1 week. Post-ablation fever was encountered in 13 patients, but the temperature of 12 of the patients was lower than 38 °C. The last patient had peritonitis and the temperature reached 41.2 °C; oral antipyretic was administrated for 3 days and the patient recovered. No skin burns were observed in the treated area; however, the treated area was slightly swollen in five patients, and no extra treatment was applied.
During the follow-up period, eight patients died of primary tumor progression during 6–24 months after MWA, and two patients died of cardiovascular at 7 months after MWA and cerebrovascular at 10 months after MWA. In the other patients, the ablation zones were well defined on contrast-enhanced imaging and gradually shrank with time.
21.7 Comparison with Other Treatments
Local recurrence and distant metastasis present a great challenge for treating malignant tumors after conventional surgery, radiotherapy (external beam radiotherapy), and chemotherapy. Effective treatments are needed to prolong survival and improve the quality of life for recurrent or metastatic superficial tumors.
Surgical resection is the most invasive method for superficial tumors, but open surgery brings about the risk of hemorrhage and possible postoperative incision hernia. Meanwhile, if the resection margin is inadequate, reoperation of SMTs can significantly reduce immunity and increase the chance of recurrence and metastasis. Some patients may not be surgical candidates due to poor medical condition [19]. Reoperation of SMTs can significantly reduce immunity and increase the chance of recurrence and metastasis; sometimes surgery provides only temporary treatment for recurrent or metastatic SMTs without prolonging survival time [20].
Minimal invasive treatments are suitable for treating such tumors. The management of superficial malignant tumors underwent a dramatic evolution in the last decade [18, 21]. Transarterial embolization is used for tumors larger than 4 cm in diameter at present, especially for patients who are nonsurgical candidates [21]. However, superficial tumors have relatively poor blood supply than the solid ones, and it cause difficult to the embolization. In addition, it is difficult to create a safety margin to eradicate possible microscopic tumor and it may cause ischemic changes. Thus, it is rarely used in superficial tumors. The local and minimal invasive treatments must be applied to overcome these treatments preclude, which should have image precise monitoring system and image-guided thermal ablation system.
HIFU ablation is a noninvasive technique for the treatment of localized tumors [22, 23]. HIFU is a conformal extracorporeal treatment which can generate thermal coagulation necrosis of the target lesion without surgical exposure or insertion of applicators. There have been many clinical studies using HIFU for alleviation of solid tumors recently and has obtained effective local tumor control [24–27]. In the treatment of superficial tumors, it also achieved a satisfying result, with the technique effectiveness ranging from 57.9 to 100 % [28–30]. But it also has some limitations; HIFU treatment takes more time, hence increasing the incidence of complication, and ablation is influenced by the acoustic pathway, which cannot treat undetectable tumors.
MWA, which is the newer technique, is technically similar to radiofrequency ablation. Radiofrequency ablation remains the most widely used ablative technique [31–33], and MWA has its own advantages. Microwave power can be continually applied to produce extremely high (>150 °C) temperatures, which improves ablation efficacy by increasing thermal conduction into the surrounding tissue [34]. Microwaves also heat tissue more efficiently than does radiofrequency energy, microwaves do not require ground pads, and multiple antennas can be operated simultaneously [35]. But it is unable to compare the pros and cons among these treatment methods in the treatment of SMTs, for it has relatively limited studies and lacking of systemic and strict controlled studies.
125I seed implant brachytherapy is a relatively new radiotherapy treatment; it has become one of the standard treatments of prostate cancer [36, 37]. With the improvement of the technique, 125I seed implant brachytherapy has been used for various kinds of tumors [38–40]; however, no report assesses the efficacy and safety of 125I seed implant brachytherapy for SMTs; it can be used as an adjunctive therapy during MWA.
21.8 Technique Key Points
On account of the previous experience in MWA for solid tumors, the ultrasound-guided MWA was used for the SMTs. The treatment effectiveness was 93.1 %, and 54 of 58 lesions were successfully treated. Tumor size was an important factor to influence the efficiency. When the tumor diameter was <4 cm, the tumor effectiveness was 97.9 %; however, when the tumor diameter was ≥4 cm, the effectiveness was 75 %. This might result from an inadequate ablation dosage, and the larger tumors have more relatively abundant blood supply. In order to achieve the same effect, tumors larger than 4 cm needed several sessions. Results suggest that microwave may be effective for superficial tumors like other techniques; it may also represent a competitive alternative to surgical and other therapies.
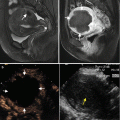
Some important points need attenting during the ablation: (1) Though an antenna is sufficiently for tumors less than 1.5 cm, when the tumors have abundant nourishing vessel, another antenna is required to successfully treat the entire tumor and ablative margin. In order to improve the ablation efficiency, we also made some progress with the antenna. Three types of antenna were used in this study; according to the tumor size and location, the antenna tips were 0.5, 0.7, and 1.1 cm, respectively. Based on the preliminary experiments, the reform antenna tip of 0.7 and 0.5 cm can ablate smaller zones than the common tip of 1.1 cm, which can be used for the superficial tumors, especially for the smaller ones. (2) It must be concerned in and after the ablation for the older patients or the patients with hypertension medical history, cardio-cerebrovascular condition status. (3) Enough rehydration salt is needed for the patients with abnormal renal function to avoid the renal insufficiency or acute renal failure. Microscopic hematuria must be observed after ablation. (4) Palliative ablation is taken for tumor larger than 4 cm to reduce the tumor burden. The whole tumor could not be ablated in one treatment session. The larger ablation zone we ablated, the more complications for patients. The biggest or the remarkable clinical symptoms of tumor are first treated during ablation to alleviate the clinical symptom. The patient needs to be discharged from the hospital for a period (usually 1 to 2 months) when ablating a number of tumors, and further treatment is administered if necessary. (5) Patients with primary tumors that are prone to develop in multiple organs are unlikely to be successfully treated with local therapy; regardless of how successfully individual lesions are destroyed. Treatment failure in these cases is due largely to uncontrolled growth of previously undetected metastases. Systemic treatment and close follow-up are needed for all patients, especially for those with recurrent or metastatic tumors. (6) Sometimes, for the tumor which is relatively superficial or it have special structure of the skin, the skin edema, hematoma and even burn will be account during and after the MWA for SMTs. The patients skin edema and hematoma will be relief from the cold compresses and the use of repercussive after MWA; (7) Combining therapies (such as chemotherapy and radiation) could be used in MWA; it acts with synergistic effects which are able to reduce the ablative margin (Figs. 21.1 and 21.2).


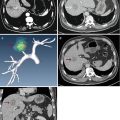
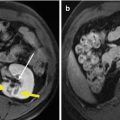
Fig. 21.1




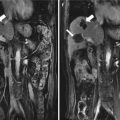
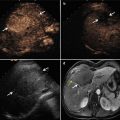
Microwave ablation (MWA) in a 71-year-old man with right abdominal wall metastatic tumor, post operation of colon cancer, splenectomy of spleen metastasis, and radiofrequency ablation (RFA) for the liver metastasis. (a) Pre-ablation arterial-phase magnetic resonance imaging (MRI) scan shows one round-like enhancement neoplasm (arrow) near the right abdominal wall with the size of 2.2 × 1.4 cm. (b) Contrast-enhanced ultrasound shows no enhancement of the ablation zone at 3 days after treatment. (c) Arterial-phase MRI scan obtained 2 months after ablation shows hypoattenuating ablation zone (arrow) without enhancement. (d) Scan obtained 6 months after ablation shows hypoattenuating ablation zone (arrow) without enhancement corresponding to treated region
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree