General Considerations
General Considerations
After informed consent, a thorough ultrasound examination of the patient’s area of complaint is mandatory before beginning any procedure to better delineate the clinically suspected pathology and to localize it precisely with respect to adjacent structures. This allows planning of the entry site and the orientation of needle placement. The patient should be positioned appropriately to ensure the most comfortable procedure for both the physician and patient. Surrounding structures of the target should be kept in a relaxed position to decrease resistance during needle insertion.
Although the risk of complications (e.g., septic arthritis) is low, disinfection of the patient’s skin and the instruments is performed. The surrounding area should be covered with a sterile drape and only the field of interest should remain uncovered to minimize the risk of contamination. Other critical issues in musculoskeletal interventions are existing coagulative disorders and anticoagulant treatment. However, bleeding diathesis represents no absolute contraindication in most cases where needles are used for the procedure. If large needles have to be used, coagulation tests including prothrombin time, international normalized ratio (INR), and a platelet count should be performed at least 24 hours before the intervention.
The ultrasound scanner should be fitted with high-frequency probes (10–15 MHz or higher) for superficial regions (hands, feet, elbow, shoulder) and with lower frequencies for deeper structures such as the knee, hip, and sacroiliac joint (5–12 MHz). A small footprint probe may allow better access to small superficial structures and an easier handling of the probe and the syringe. Normally, no needle-guidance kit is required because a freehand technique allows for a faster and more flexible (no fixed angle) intervention. The shortest needle path should be selected, avoiding vessels, nerves, and tendons, and the entry point may be marked on the skin with an indelible ink marker. After entering with the needle under the skin, sterile ultrasound gel should be used for better real-time visualization of the needle.
 Biopsy
Biopsy
Biopsies of soft tissue masses are required when imaging does not allow for a definite characterization of the lesion. Lipoma, sarcoma, desmoid, nodular fasciitis, metastatic lesions (Fig. 13.1), lymphoma, and epidermal cysts (Fig. 13.2) are some common examples where biopsy might be necessary for a better differentiation, and ultrasound-guided biopsy of masses in the musculoskeletal system can be acquired quickly, accurately, and safely. The most common complications of surgical biopsies, including wound infection, local pain, hematoma, seroma, keloids, and problems related to general anesthesia, are almost nonexistent in image-guided percutaneous biopsies. The reported complication rate of image-guided percutaneous biopsies is between 0 and 0.5%. However, malignant masses can spread along the needle track (e.g., sarcoma); therefore, an appropriate needle pathway should be discussed with the surgeons before the intervention. Considering compartment anatomy is critical in tumor biopsies to avoid seeding into ulterior compartments, which also might be resected after histologic confirmation. Fine needle aspiration, moving end-cutting 20- or 22-gauge spinal or Chiba needles up and down inside the mass, is used for cytologic examination and might be sufficient for diagnosis. However, most solid masses require larger needle biopsies to provide samples for histologic assessment.
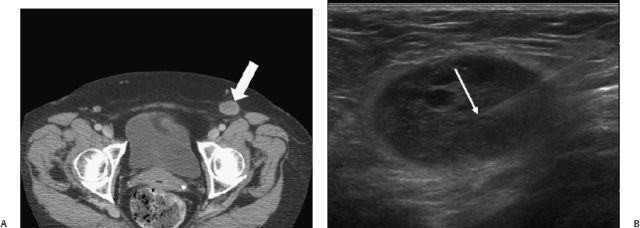
Fig. 13.1 In a 70-year-old woman with a history of colon carcinoma, a suspicious left groin node was detected on computed tomography (A) (arrow). (B) Ultrasound examination was suspicious for a pathologic lymph node and fine needle aspiration confirmed metastatic colon carcinoma (arrow).
For local anesthesia, a solution of lidocaine at a concentration of 1 to 2% can be used, and bicarbonate can be added to prevent a burning sensation on the skin. Using the freehand technique with a long axis view of the needle, a 14-to 18-gauge automatic core biopsy needle may be used to gather multiple core samples (3–6) for each biopsy, which are then placed in formaldehyde solution. After reaching the lesion with the tip of the needle, it should be remembered that after activation the needle will advance an additional 18 to 19 mm into the lesion, and care should be taken to preserve soft tissue structures that are located dorsally to the lesion. Therefore, surrounding tissue has to be assessed routinely before the procedure, and color Doppler ultrasound allows visualization of viable tissue and adjacent vessels.
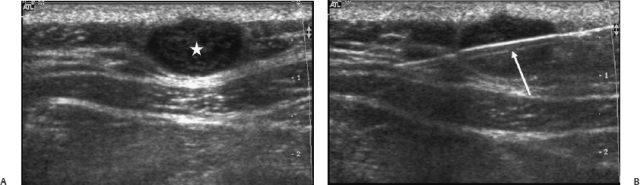
Fig. 13.2 A 49-year-old man with a soft tissue mass at the right side of the posterior neck. (A) Ultrasound showed a subcutaneous oval nodule with clear margins and inhomogeneously hypoechoic echo-texture (*). (B) The underlying muscle fascia was compressed, but not infiltrated. To rule out malignancy, a biopsy was performed under ultrasound guidance using an 18- gauge core biopsy needle (arrow). Histologic analysis of the sample was diagnostic for an epidermal cyst.
An ambulatory setting is normally sufficient except for severely ill patients and patients requiring sedation. Outpatients should be observed for at least 1 hour after biopsy. They should be discharged with stable blood pressure and no evidence of active bleeding.
Ultrasound may also be used to identify selected muscle involvement in neuromuscular disorders such as Duchenne or Becker muscular dystrophies and to guide muscle biopsy in these cases.
Analysis of synovial tissue can provide relevant information about the pathophysiologic mechanism, the degree of inflammation, and prognosis. Ultrasound-guided biopsy can be used to obtain synovial samples, but it is mainly performed for research purposes.
In summary, ultrasound-guided biopsy is a reliable technique to characterize soft tissue lesions in most cases. However, if histologic diagnosis is equivocal or negative for malignancy, but a high clinical or radiologic suspicion for malignancy still exists, open biopsy should be performed.
 Foreign Body Extraction
Foreign Body Extraction
Identification of soft tissue foreign bodies is routinely performed by using radiographs. However, ultrasound is able to detect and assess a variety of foreign bodies of different shapes and material compositions, with the added advantage of being able to detect nonradiopaque foreign bodies such as wood, plastic, and often glass. Foreign bodies appear mainly as a hyperechoic structure surrounded by hypoechoic granulation tissue. Ultrasound can be used for preoperative localization of the foreign body, and also to guide the extraction process and avoid surgery.
Ultrasound-guided removal of superficial foreign bodies can be performed more easily when the compositions of the fragments are metallic splinters (Fig. 13.3) or gravel, but removal of pieces of glass (Fig. 13.4) and wood (Figs. 13.5 and 13.6) require more patience and meticulous technique. Besides adequate local anesthesia, a set of sterilized surgical instruments, including Mosquito, Kelly, Kocher, and Splinter forceps is needed. After marking the position of the best extractable side of the foreign body, a skin incision is usually necessary to allow the instrument to enter the soft tissue and to proceed toward the foreign body. When an open wound is present, it may be possible to extract the foreign body through the same path that it entered. If an incision is necessary, care should be taken to ensure an adequate incision width to allow extraction of the foreign body without significant injury to adjacent soft tissues. Real-time ultrasound guidance allows for a simpler extraction procedure, as it is easier to estimate the location and angle at which the foreign body is lying.
 Calcification Puncture and Lavage
Calcification Puncture and Lavage
Calcific tendinitis is caused by calcium hydroxyapatite deposition, which can theoretically affect all tendons, but it is found most commonly in the rotator cuff tendons of the shoulder. Predominantly, the supraspinatus tendon is involved either unilaterally or bilaterally in 30- to 60-year-old patients, with a higher incidence range in women (57–76.7%). Although calcific tendinitis is a self-limited process in which the calcifications tend to be reabsorbed, in 50% of patients these deposits become symptomatic, causing acute or chronic pain. The disease is divided into two stages: the formative phase in which calcium hydroxyapatite is deposited into the tendon and the resorptive phase where these deposits resolve. Systemic anti-inflammatory drugs, iontophoresis, physiotherapy, and subacromial injections of corticosteroids often provide only temporary relief and these patients may benefit from removing the calcium. Surgery and high-intensity shock waves are two more invasive and relatively expensive procedures, but a simpler and more inexpensive method to remove the calcium crystals is ultrasound-guided needle puncture of the calcification followed by lavage.
Ultrasound scanning helps to localize the calcification, which appears as a hyperechoic structure with or without posterior shadowing. After local freezing, the calcification is repeatedly punctured with a 16- to 22-gauge needle under ultrasound guidance in an attempt to fragment the calcific deposit. Different methods are described for the second part of the procedure, where after injections of small amounts of 1% lidocaine or 0.9% saline using a 10- to 20-mL syringe, aspiration of the calcium is performed. Although some authors are using a single needle for puncture, injection, and aspiration, we prefer using a two-needle technique. One is inserted for injection of saline and the second one is used for aspiration; in our experience, there is less frequent occlusion of the needle using this technique. Aspiration is easier in tuff -like or semiliquid calcifications representing reabsorptive states (Fig. 13.7), but more difficult when a solid calcification is present (Fig. 13.8). In both cases, aspiration of a cloudy, milky fluid or a solid gritty substance is performed until the aspirated fluid becomes clear. Generally, only a part of the calcification can be aspirated, but this does not represent a failure of the treatment as the residual calcium tends to undergo spontaneous resorption over the weeks following the procedure (Fig. 13.9). Before extracting the needle, intrabursal injection of corticosteroids and longacting anesthetics is performed to decrease crystal-induced inflammation caused by local diffusion of the calcific material.
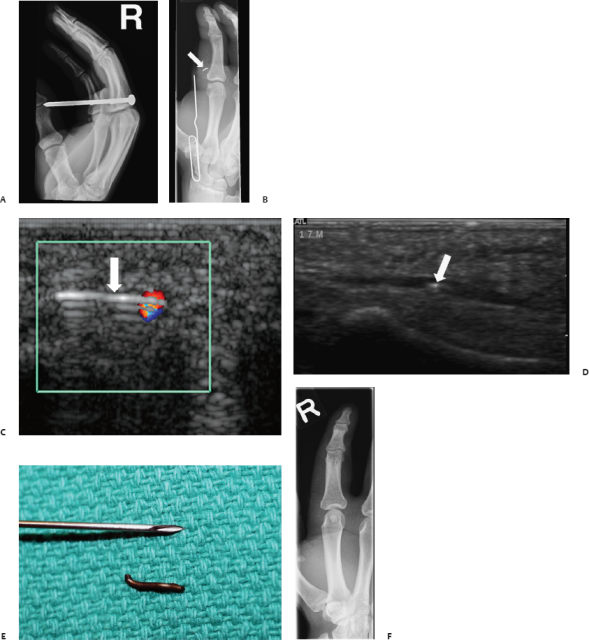
Fig. 13.3 Radiograph shows right hand of a 17-year-old man with a screw penetrating his second finger at the level of the proximal phalanx, but not penetrating the bone (A). (B-D) After extraction of the screw in the emergency department, a 7 mm foreign body piece was still detectable and ultrasound scanning detected the intravascular location of the foreign body (arrows). (C) Note the reverberation artifact, consistent with the presence of metal. (E) Photograph shows the extracted metallic foreign body. (F) Plain film radiograph confirmed complete extraction of the foreign body with some residual soft tissue swelling.
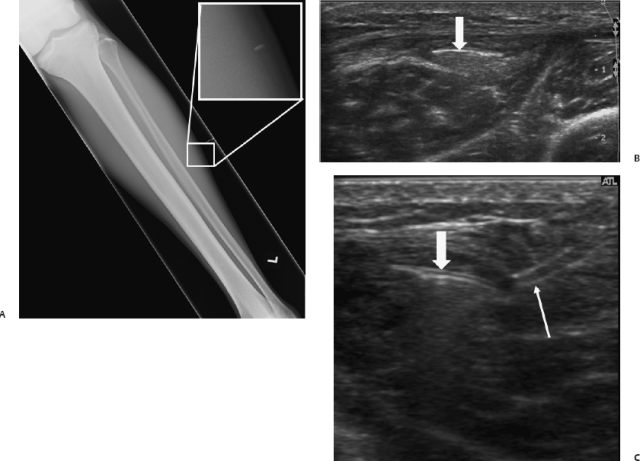
Fig. 13.4 A small foreign body was detected on radiographs in a 16-year-old boy (A).(B) By using ultrasound, visibilit y of the foreign body was distinctly improved and extraction was per formed (arrow). (C) Ultrasound image shows the foreign body (arrow) with the two grips of the forceps (thin arrow). The foreign body was made of glass, which explained the poor visibility on plain films.
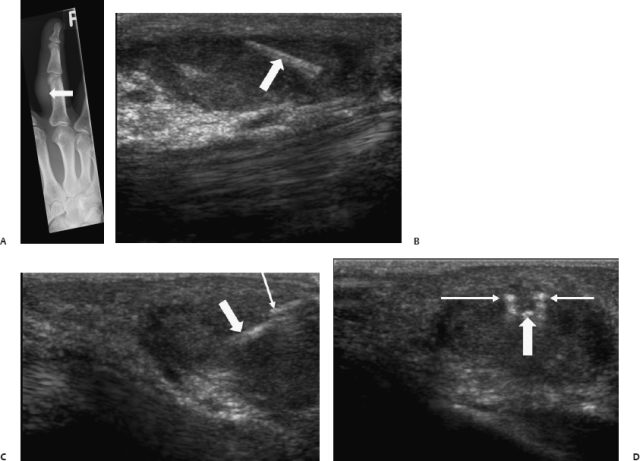
Fig. 13.5 A 53-year-old patient with a painful third right finger for 3 months after gardening. On plain films only soft tissue swelling was detectable (A) (arrow). (B) Ultrasound showed a foreign body surrounded by granulomatous tissue (arrow). Ultrasound-guided foreign body removal was performed and a thorn was extracted. (C,D) Ultrasound images show the forceps and the foreign body (arrows) on orthogonal planes.
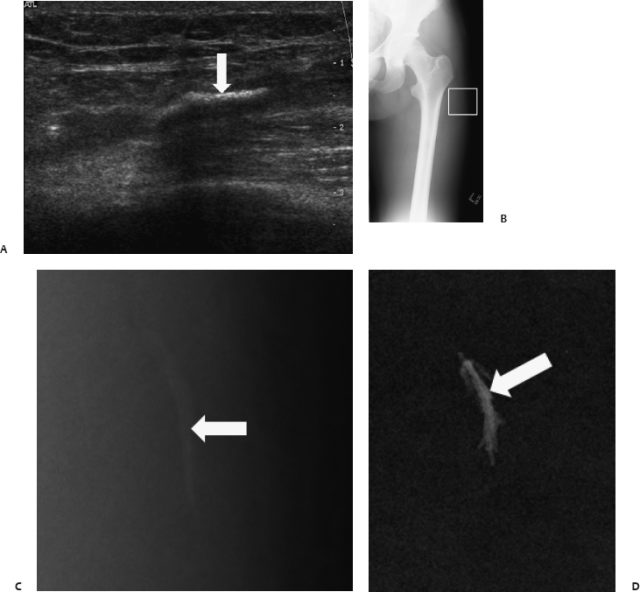
Fig. 13.6 After three episodes of local infection in an 18-year-old male patient, a foreign body was detected in the postero-lateral thigh on ultrasound (A). (B,C) On radiographs, the same lesion was barely seen (arrow). (D) After ultrasound- guided removal, fluoroscopy of the specimen was performed showing a calcified splinter (arrow).
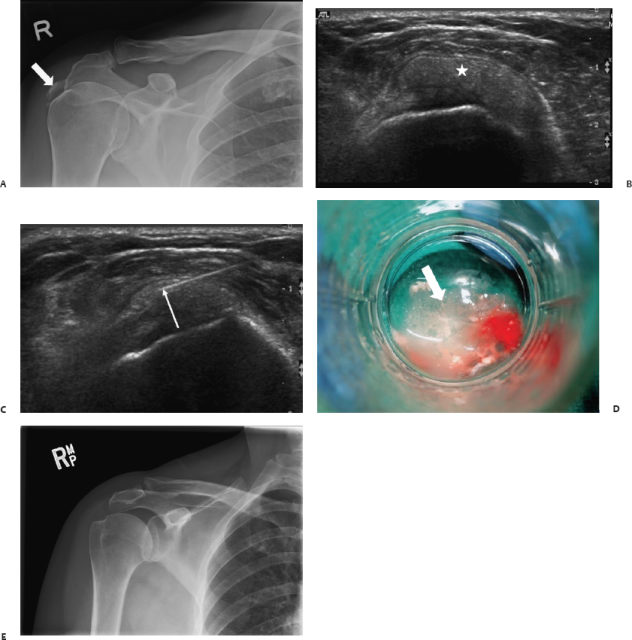
Fig. 13.7 A 58-year-old woman was referred with functional impairment of the shoulder. (A) A plain radiograph showed a large calcification at the level of the supraspinatus tendon (arrow). (B) Ultrasound showed a “soft” intratendinous calcification without posterior shadowing, normally representing the reabsorptive stage of the disease (*). (C,D) Toothpaste-like material was aspirated under ultrasound, followed by lavage and steroid injection into the subdeltoid bursa (arrows). (E) Three months after the procedure radiographs showed complete resolution of the calcification.
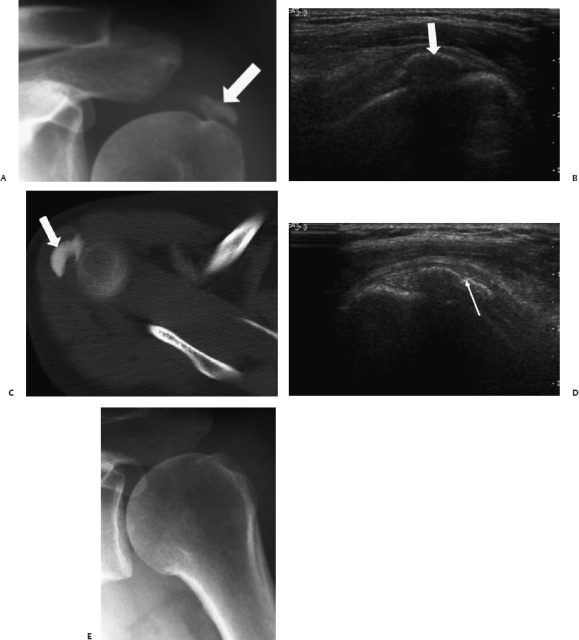
Fig. 13.8 Shoulder lavage. (A) Radiographs (B) ultrasound (C) and computed tomography scan showed a large calcification in the supraspinatus tendon in a male patient (arrows). (D)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree