Neeral R. Patel, James P.F. Burn, and Ali Alsafi Department of Imaging, Imperial College Healthcare NHS Trust, London, UK Over the past three decades, ultrasound has become widely available, with increasingly powerful and portable machines at lower cost. Ultrasound is now more accessible across healthcare settings. In addition to its use as a diagnostic modality, guidance on minimally invasive procedures has been adopted widely in interventional radiology as well as other invasive disciplines. This has improved technical success and resulted in a proven reduction in complications. This is most notable in the use of ultrasound for central venous access. As hepatobiliary intervention becomes ever more complex, with a variety of pathologies being treated in a minimally invasive manner, ultrasound guidance is now indispensable. In this chapter we will discuss the use of ultrasound in some common hepatobiliary interventional procedures. Prior to any interventional procedure, patients’ clinical history and previous imaging should be reviewed. Reviewing previous imaging gives the operator the opportunity to assess suitability for ultrasound guidance as opposed to other modalities such as fluoroscopy or computed tomography (CT). Cross‐sectional imaging is particularly useful in planning intervention, but is not always necessary. The indications and appropriateness of the procedure should be carefully considered. For intervention in very ill or uncooperative patients, general anaesthesia should be considered. A comprehensive approach to preparing any patient for an ultrasound‐guided procedure can be remembered with the acronym ABCD: Written informed consent is imperative in order for the operator to proceed, and should be obtained by explaining to the patient, in lay terms, what the procedure will entail, the risks and the benefits, and alternative options, as well as satisfying any questions or concerns the patient may have. In circumstances where the patient is unable to give informed consent or does not have capacity, local ethical guidance should be applied. Prior to any ultrasound‐guided liver procedure, the patient’s blood results must be scrutinised, in particular the clotting profile, to minimise the risk of bleeding. The acceptable limit will depend on local guidance and the level of risk of the planned intervention. The majority of ultrasound‐guided liver interventions will fall under the moderate‐risk category (liver biopsy, drain/aspiration, simple radiofrequency ablation [RFA]), while biliary drainage and complex RFAs are considered high‐risk procedures. An example of the recommendations for blood result thresholds and pausing anticoagulants (based on Society of Interventional Radiology [SIR] guidance) when planning ultrasound‐guided liver procedures is summarised in Table 14.1 [1]. Table 14.1 Guidance on coagulation threshold and pausing anticoagulants based on the bleeding risk of the ultrasound‐guided liver intervention being performed. *duration to withhold depends on INR levels prior to biopsy A safety checklist undertaken with all members of staff involved in the procedure is essential to reduce the risk of untoward events [2]. A modified World Health Organization (WHO) surgical checklist, specific to image‐guided intervention, may be used [3]. These include prophylactic antibiotics, analgesia, and conscious sedation medication. There is joint Cardiovascular and Interventional Radiological Society of Europe (CIRSE) and SIR guidance on the use of antibiotics in interventional radiology, which includes their use prior to intrahepatic abscess and biliary drainage as well as RFA. The exact antibiotic(s) to be administered will depend on local antibiotic policy and sensitivities. It is important that prophylactic doses are administered within 60 minutes of skin puncture [4]. Liver intervention can be complex and often lengthy. For this reason, conscious sedation and analgesia are used to improve patient tolerance. Titrated doses of midazolam and fentanyl used in combination can provide effective conscious sedation. In procedures such as RFA of liver lesions, where high doses of sedation are likely to be required, some operators prefer the use of general anaesthesia, with the added advantage of reproducible breath holding for accurate needle positioning. Whichever is chosen, the patient must be fasted for at least 4–6 hours prior to the procedure. Percutaneous liver biopsy is the standard procedure for obtaining liver tissue for histopathological assessment, used principally in the diagnosis and management of parenchymal liver diseases (‘non‐targeted’) and in the characterisation of liver lesions (‘targeted’). A ‘blind’ liver biopsy is obtained without imaging during, or immediately prior to, taking the biopsy. This has largely been superseded by image‐guided biopsy, with a reduction in major complications, post‐biopsy pain, and biopsy failure [5]. The reported risk of a major complication resulting from percutaneous liver biopsy remains 0.6–2%. Ultrasound is the preferred modality, as it provides real‐time imaging in a non‐conventional plane from skin to lesion. If a targeted biopsy is intended, CT or magnetic resonance imaging (MRI) within four weeks prior to the biopsy is recommended by the British Society of Gastroenterology. This is essential to assess the exact location of the target lesion, its vascularity, and the distribution of regional vessels and bile ducts, as well as the presence of extrahepatic disease. All of these will influence the risk/benefit assessment in determining whether to proceed with biopsy. Full ultrasound assessment immediately prior to biopsy is also essential. This will confirm the ongoing presence of a target lesion (e.g. one that may have responded to chemotherapy since prior imaging) and to identify any new preclusive factors, such as intervening bowel loops, large‐volume ascites, or biliary obstruction. In addition to uncorrected coagulopathy, the presence of ascites is widely considered a contraindication to liver biopsy. The exact impact of ascites on bleeding risk is not clear [6]. Despite this, given the theoretical risk of uncontrolled bleeding into the ascitic fluid, pre‐biopsy paracentesis or a transjugular route is recommended in such instances [7]. The presence of intrahepatic biliary dilatation poses a risk of biliary leak resulting in biloma or biliary peritonitis, with serious complications occurring in up to 4% of cases [8]. It is therefore prudent to consider biliary drainage and transbiliary biopsy when feasible [9]. Pre‐procedural ultrasound is required to plan optimal patient positioning for biopsy. The relationship of the liver to adjacent organs or overlying ribs may be improved from that demonstrated on prior cross‐sectional imaging by changing patient positioning, for instance left lateral decubitus of posterior right lobe of the liver lesions. Once the lesion and a safe approach have been identified (Figure 14.1), the skin is marked, cleaned, and draped in a sterile manner. The skin and deep tissues are infiltrated with local anaesthetic (typically 10–20 mL of 1% lidocaine). It is important to inject a sizeable proportion of the local anaesthetic in the region of the liver capsule, as this is well innervated and a traversing needle can cause significant discomfort to the patient if they are not adequately anesthetised. At the planned site of entry, a small nick is made in the skin with a blade. During the biopsy, respiration may be ceased in order to arrest diaphragmatic and liver movement. Typically this is in end‐expiration, both to aid consistent reproducibility of liver position and to ensure maximum elevation of the diaphragm/pleura away from the biopsy site. The specific technique thereafter varies somewhat according to whether the biopsy is targeted, needle selection, and whether a coaxial technique is employed. A variety of needle types are used for liver biopsy, each with its own merits and limitations. While the balance of patient safety and diagnostic yield is paramount, selection of technique may be influenced by operator preference, risk factors for haemorrhage, cost and availability. The Tru‐Cut® biopsy needle (Merit Medical, South Jordan, UT, USA) is a disposable needle comprising an obturator with a sharp end and outer sheath integrated into an all‐in‐one design. The device is advanced up to the margin of the target in the closed position. The obturator is then advanced through the target tissue while holding the outer cutting sheath steady. Finally, the sheath is advanced to cut the liver and the whole assembly is withdrawn. The Tru‐Cut biopsy needles have been superseded by automatic and semi‐automatic variants, which are spring‐loaded devices. Automatic needles trigger a rapid‐firing side‐notched Tru‐Cut–type biopsy needle, while in semi‐automatic devices the obturator is advanced manually as an isolated action before triggering the rapid‐fire outer sheath. The latter has the advantage of allowing real‐time assessment of the full length of the needle, with the ability to readjust position prior to firing. This is particularly important when the deep margin of the lesion poses a risk. This method is favoured by the authors. More recently, an end‐cut full‐core needle such as the Biopince™ (Argon Medical, Athens, TX, USA) spring‐loaded automatic needle has been shown to improve the diagnostic yield by increasing core length and reducing crush artefact [10]. The biopsy yield must be counterbalanced by safety considerations when the deep margin is threatened by a large vessel, duct or diaphragm. Figure 14.1 (a, b) Pre‐procedure ultrasound with and without annotations demonstrating a hypoechoic lesion in segment II of the liver. (c, d) Ultrasound of the left lobe of the liver with a biopsy needle (arrowheads) in the target lesion with and without annotations. Use of aspiration needles such as Jamshidi™ (Becton, Dickinson, Franklin Lakes, NJ, USA), Klatskin, and Menghini is declining, but was reserved for non‐targeted parenchymal biopsies. The needles are an open core with either an internal or external bevel, which is passed into liver tissue and then withdrawn while steady suction is applied to a connected syringe. The specimen is separated distally from the liver by the power of suction. The technique is associated with a reduced risk of haemorrhage but variable core quality [11]. A coaxial technique may be used for biopsy of a focal lesion. This entails image‐guided positioning of an outer guide needle (Figure 14.2; at least one gauge larger than the actual biopsy needle), within or adjacent to the lesion. A second biopsy needle is then placed through the guide needle to obtain samples. The method allows multiple samples to be obtained without having to repeatedly reaccess the lesion, or indeed puncture the liver capsule. The theoretical impact on safety, however, has not been proven [12]. Figure 14.2 (a) Semi‐automatic Tru‐Cut–style biopsy needle. (b) Biopsy needle with the specimen obturator fully advanced prior to clicking the plunger and deploying the cutting cannula (note the specimen notch). (c, d) Coaxial introducer needle before and after removal of the stylet.
14
Ultrasound in Hepatobiliary Intervention
Patient Preparation
Authorisation (Informed Consent)
Blood Results and Blood Thinners
Low risk
Moderate risk
High risk
Example cases
Ultrasound‐guided abdominal paracentesis
Liver biopsy (targeted and non‐targeted)
Intrahepatic abscess drain/aspiration
Radiofrequency ablation of a liver lesion (simple)
Biliary drainage
Radiofrequency ablation of a liver lesion (complex)
Haemoglobin
>80 g/L
>80 g/L
>80 g/L
Platelets
>50 × 109/L
>80 × 109/L
>80 × 109/L
Prothrombin time (PT)
<25 seconds
<24 seconds
<22 seconds
International normalised ratio (INR)
<1.6
<1.5
<1.4
Activated partial thromboplastin time (APTT)
<42 seconds
<41 seconds
<40 seconds
Aspirin
Continue
Continue
Continue
Abciximab
Withhold for 2 days
Withhold for 2 days
Withhold for 2 days
Clopidogrel
Withhold for 5 days
Withhold for 5 days
Withhold for 5 days
Direct thrombin inhibitors (e.g. dabigatran)
Withhold for 2 days
Withhold for 2 days
Withhold for 2 days
Factor Xa inhibitors (e.g. apixaban)
Withhold for 2 days
Withhold for 2 days
Withhold for 2 days
Low molecular weight heparin (e.g. enoxaparin)
Withhold prophylactic dose for 12 hours; therapeutic dose for 24 hours
Withhold prophylactic dose for 12 hours; therapeutic dose for 24 hours
Withhold prophylactic dose for 12 hours; therapeutic dose for 24 hours
Warfarin*
Withhold for 3 days
Withhold for 3 days
Withhold for 5 days
Checklist
Drugs
Liver Biopsies
Pre‐procedural Imaging
Consideration of Contraindications
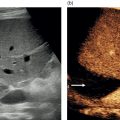
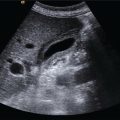
Technique
Needle Choice
Cutting‐Type Needles

Aspiration or Suction‐Type Needles
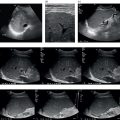
Coaxial Technique

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree