Ultrasound of Organ Transplants and Transjugular Intrahepatic Portosystemic Shunt
Renal Transplants
Sonography plays a central role in the initial evaluation and long-term follow-up of renal transplants. US is used to detect early and late complications and to guide aspiration of fluid collections and biopsies of the transplanted kidney. Doppler US is used to screen for vascular complications, which are then usually confirmed and often treated by angiographic techniques [1,2].
Surgical Technique
Transplant kidneys are most often placed in the iliac fossa in a retroperitoneal position. When combined with pancreas transplantation, the renal transplant is intraperitoneal in position. The renal pelvis and ureter are routinely positioned anteriorly to allow for easier access if subsequent intervention is necessary. For this reason a right donor kidney is usually turned over and placed in the recipient’s left iliac fossa. A left donor kidney is usually placed in the right iliac fossa. The transplant renal artery may be connected to either the internal or external iliac artery. End-to-side anastomosis to the external iliac artery is often preferred because of a lower incidence of postoperative stenosis. Cadaver kidneys are commonly
removed with the renal artery intact and still attached to a portion of the aorta. The aorta is trimmed to an oval or round shape (a Carrel patch) and is sutured end-to-side to the external iliac artery of the recipient. Arteries of living donor kidneys are harvested without an aortic patch and are anastomosed directly to the recipient artery. Connection to the internal iliac artery is made end-to-end. Multiple renal transplant arteries require additional anastomoses. The transplant renal vein is connected end-to-side to the external iliac vein with special care taken to ensure that the vein is mobile and is not kinked. The transplant ureter is usually connected to the bladder directly by ureteroneocystostomy. Occasionally the transplant ureter is connected to a native ureter or to an ileal conduit. US examination is commonly performed within the first 24 hours to provide a baseline scan for comparison with future studies.
removed with the renal artery intact and still attached to a portion of the aorta. The aorta is trimmed to an oval or round shape (a Carrel patch) and is sutured end-to-side to the external iliac artery of the recipient. Arteries of living donor kidneys are harvested without an aortic patch and are anastomosed directly to the recipient artery. Connection to the internal iliac artery is made end-to-end. Multiple renal transplant arteries require additional anastomoses. The transplant renal vein is connected end-to-side to the external iliac vein with special care taken to ensure that the vein is mobile and is not kinked. The transplant ureter is usually connected to the bladder directly by ureteroneocystostomy. Occasionally the transplant ureter is connected to a native ureter or to an ileal conduit. US examination is commonly performed within the first 24 hours to provide a baseline scan for comparison with future studies.
Renal Transplant Scan Protocol
Because the renal transplant is usually superficial, a high-frequency 5.0-7.0-MHz curved array transducer is preferred. For larger patients with deeper transplant kidneys, a 3.5-4.0-MHz sector transducer may be used. The kidney is scanned in its long and transverse axes. Measurements are made of its greatest length, width, and anteroposterior (AP) dimensions. Renal volume is calculated by the standard formula (volume = length × width × AP dimension × 0.523). Renal size is an important parameter to follow to assess for pathologic changes. The echogenicity of the cortex, medulla, and renal sinus is carefully evaluated and compared to images from previous US examinations. The perirenal areas are scanned for fluid collections, hematomas, solid masses, and adenopathy. A full bladder is preferred to displace overlying bowel out of the pelvis and away from the transplant. If hydronephrosis is present, the bladder should be emptied to see if the dilatation is caused by bladder distention. A dilated ureter should be searched for.
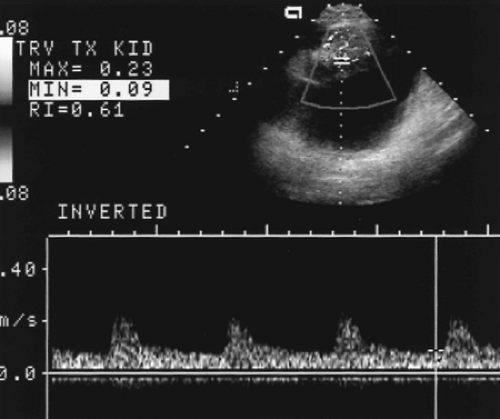
Patency of the transplant arteries and vein should be confirmed with color and spectral Doppler. Spectral waveforms of intrarenal arteries at the upper pole, mid-kidney, and lower pole are obtained and resistance index (RI) calculated from the waveforms. Intrarenal arteries are identified by flashes of color on color flow imaging. A small spectral Doppler sample volume is placed over the flashing color signal and is moved until an adequate spectral tracing is obtained. Because the intrarenal arteries are usually not discretely visualized, no attempt is made to determine Doppler angle. Power Doppler provides evaluation of vascular perfusion of the transplant kidney and is used to detect focal flow defects.
US imaging is highly cost-effective as a screening tool to detect complications. Examinations should be interpreted with a low threshold for abnormalities, which are then confirmed by additional testing [1].
Normal Renal Transplant
The following features characterize a normal renal transplant:
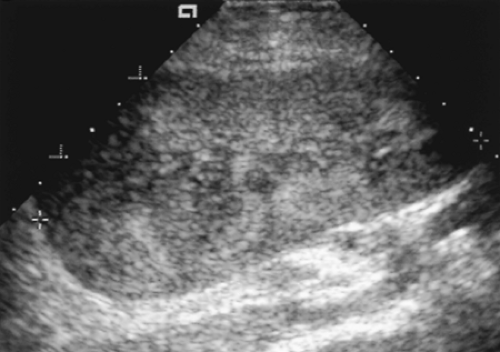
The echogenicity of the renal cortex is equal to or slightly greater than the echogenicity of the medulla. Absence of corticomedullary differentiation is a normal finding seen in many transplants.
The echogenicity of the cortex and medulla is uniform throughout the kidney. Focal areas of increased or decreased echogenicity suggest edema, hemorrhage, infection, ischemia, or infarction.
Renal size measurements are compared to previous scans. In transverse plane the kidney is elliptical in shape with the width being greater than the AP dimension. Rounding of the kidney with AP dimension equal to transverse dimension is a sign of diffuse renal swelling. However, the volume of normal renal transplants may increase up to 20% in the first 2-3 weeks after transplantation [3].
The renal sinus should be obvious and is distinctly more echogenic than the renal parenchyma.
Mild pelviectasis is a common and usually normal finding. Calyectasis is a relatively reliable sign of obstruction.
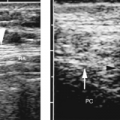
Doppler of the normal renal artery shows a low resistance pattern with forward flow into the kidney throughout the cardiac cycle. The range of normal peak systolic velocity is broad (60-203 cm/sec, mean 132 cm/sec) [4].
Renal vein flow is flat with low velocity and flow away from the kidney.
RI of intrarenal arteries is normally <0.80 (Fig. 14.1).
Complications of Renal Transplantation
Peritransplant Fluid Collections
Fluid collections adjacent to the transplant are very common (approximately 40% of transplants) and may be lymphoceles, urinomas, seromas, hematomas, or abscesses [6]. US determines the size, location, and appearance of the fluid collection. Although each entity shows some characteristic findings, differentiation is confirmed by US-guided aspiration of the fluid. Small fluid collections are often observed and usually resolve over time without compromising the transplant.
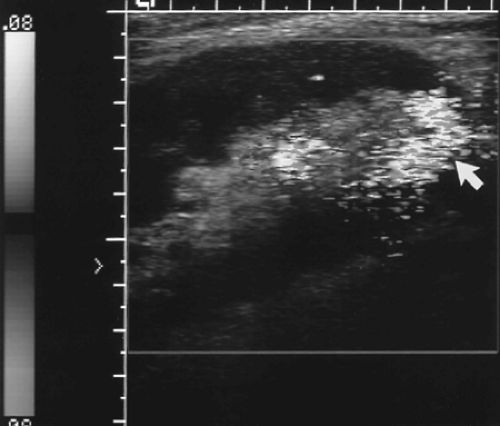
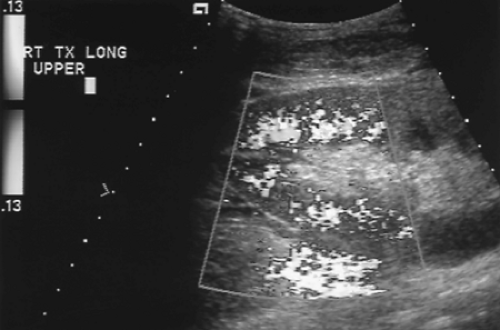 Figure 14.2 Normal Color Doppler of Renal Transplant. Color Doppler of a normal well-perfused renal transplant shows color signal well into the peripheral parenchyma (see Color Figure 14.2). |
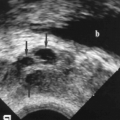
Lymphoceles are most common, affecting up to 15% of transplants. They are accumulations of lymph in the extraperitoneal space and usually result from interruption of the recipient’s lymphatic channels.
– Fluid collections are anechoic, often large in size, and most (80%) have thin septations (Fig. 14.3).
– Lymphoceles are most common 4-8 weeks after transplantation.
– Small lymphoceles are usually asymptomatic and require no therapy.
Hematomas and seromas are common but usually small and insignificant in the immediate postoperative period. Large hematomas may result from injury to the vascular pedicle of the kidney or rupture of the transplant.
– Acute hematomas are hypoechoic, solid, and often lobulated perirenal collections (Fig. 14.4).
– An increase in size of the hematoma indicates continued bleeding and a need for intervention.
– Old hematomas are most variable and show solid-appearing areas of clotted blood and cystic areas of seroma. Septations are common.
Abscess is usually an early complication (first 3 weeks), presenting with postoperative fever and pain.
– US shows a fluid collection of mixed echogenicity with internal echoes representing pus and debris.
– Gas within the fluid collection is highly indicative of infection.
Urinoma is also usually an early complication but is relatively rare. Urine leaks may develop due to faulty anastomosis or be caused by ischemic necrosis of the collecting system. Ischemic necrosis most commonly involves the distal ureter, but may involve the entire ureter and collecting system.
– The collection is purely cystic, anechoic, and usually without septations (Fig. 14.5).
– Large urinomas may cause urinary obstruction.
– Large urinomas require aspiration or drainage.
– Small urinomas regress spontaneously.
– Radionuclide imaging shows progressive accumulation of radiotracer in the collection when a urine leak is present.
In women, ovarian cysts must be considered as an additional cause of a peritransplant fluid collection.
Diminished Renal Function
Elevation of serum creatinine is a common clinical problem indicative of impaired transplant function. Causes are listed in Box 14.1. US is used to detect urinary obstruction, pathologic fluid collections, and vascular complications. However, the most common causes are acute rejection, acute tubular necrosis, and cyclosporine toxicity.
US findings are non-specific. The following findings may be evident with each of the conditions listed. US-guided core biopsy is usually performed to confirm the diagnosis.
– The kidney is often enlarged and has increased thickness of the cortex. The AP diameter is equal to or exceeds the transverse diameter.
– Pyramids may be prominent or corticomedullary differentiation may be lost.
– The renal cortex may show increased or decreased echogenicity (Fig. 14.6).
– The walls of the collecting system may be thickened.
– The central renal sinus may be effaced and its normal high echogenicity is absent.
– RI of the intrarenal arteries >0.8 is a nonspecific finding of renal dysfunction. Occasionally reversal of blood flow direction is seen in diastole.
Acute tubular necrosis results in the postoperative period from prolonged ischemia and reperfusion injury. Cold storage of the donor kidney for longer than 24-30 hours increases the probability of acute tubular necrosis. The condition is uncommon when the kidney is obtained from a living related donor.
Acute rejection occurs in the first year in up to 50% of transplant recipients. Presentation includes malaise, fever, and a painful kidney. However, cyclosporine therapy may mask all symptoms. Acute rejection is rare immediately post-transplant but usually becomes apparent on serial follow-up. Acute rejection is rare after the first year.
Chronic rejection is the most common cause of late transplant loss. Loss of renal function is progressive to complete transplant failure.
– US shows diminished renal size, thinning of the cortex, and often mild hydronephrosis.
Drug nephrotoxicity may be caused by many of the drugs used to suppress rejection, but cyclosporine toxicity is most common. Toxicity is dose dependent and function often improves with reduction of dose.
Vascular Complications
Vascular complications are an uncommon (<10%) but important cause of impaired graft function [7]. They have a high incidence of morbidity and mortality but are usually easily repaired when detected. US with spectral Doppler and color flow imaging is used to screen the transplant for vascular complications. Positive screening studies are routinely followed by angiography for confirmation and therapy.
Renal artery stenosis is the most common vascular complication (1.5-4.0% of transplants). Patients present with an audible bruit or severe hypertension unresponsive to
treatment usually within the first 3 years after transplantation. Moderate hypertension due to non-renovascular causes is common (65% of transplant patients), and usually not a sign of renal artery stenosis. Stenosis at the anastomosis is usually related to surgical technique, but stenosis anywhere along the renal artery may be caused by rejection [8].
– The renal artery is examined throughout its length with color and spectral Doppler looking for zones of high velocity flow and for zones of aliasing on color Doppler.
– Peak systolic velocity >200 cm/sec suggests significant stenosis.
– Velocity gradient >2:1 between stenotic and prestenotic segments indicates significant stenosis.
– Marked turbulence is seen downstream from the area of narrowing.
– Tardus parvus waveform is highly indicative of significant stenosis but is often not present. Identification of this waveform is important because the entire course of the artery is not always seen.
Renal vein thrombosis is rare and usually occurs in the first week after transplantation. Renal vein thrombosis commonly results in infarction that necessitates removal of the transplant.
– The kidney is hypoechoic and swollen because of edema.
– The arterial waveform shows reversed flow throughout diastole.
– No venous flow is present.
Renal artery occlusion occurs in the early postoperative period. Infarction leads to graft loss. The surgical anastomosis is usually faulty.
– No arterial or venous flow is detected by Doppler US.
– Severe acute rejection may mimic this finding. If in doubt, an arteriogram should be performed.
Intrarenal arteriovenous fistula is a complication of biopsy of the transplant when an adjacent artery and vein are lacerated. Small fistulas are incidental and resolve spontaneously. Large fistulas may shunt so much blood that the transplant becomes ischemic.
– Most arteriovenous fistulas are not evident on gray-scale US.
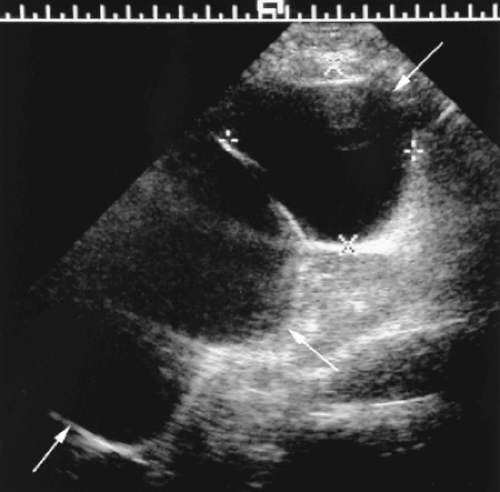
– Color flow shows turbulence, aliasing, and tissue vibration artifact in the area of the fistula (Fig. 14.7).
– High-velocity, low-resistance waveforms are seen on the arterial side.
– Draining veins show arterial pulsations.
– Tissue vibration artifact, caused by the turbulent blood flow, is shown with high sensitivity by power Doppler [9].
– Rupture of the fistula results in a large perirenal hematoma.
Pseudoaneurysms result from isolated arterial laceration during biopsy.
– Pseudoaneurysms appear as a bubble-like mass within the parenchyma.
– Doppler confirms turbulent blood flow within the mass.
– The neck of the pseudoaneurysm connecting to the injured artery may be seen when the pseudoaneurysm is large.
Urinary Obstruction
Urinary obstruction can be found during sonography for a failing renal transplant. In the immediate postoperative period, temporary obstruction may result from edema at the ureterovesical anastomosis or temporarily impaired peristalsis of the transplanted ureter [6,10].
Mild hydronephrosis (splaying of the pelvis without calyectasis) is common and is most likely due to non-obstructive causes such as overhydration or bladder distention [1].
Moderate (dilated pelvis and calyces) to severe (marked distention of the pelvis and calyces) hydronephrosis is a reliable sign of significant urinary obstruction [1]. Obstruction should be confirmed by excretory urography or radionuclide renography [11].
A dilated ureter should be searched for to determine the location of obstruction.
Peritransplant fluid collections, adenopathy or mass associated with posttransplantation lymphoproliferative disorder (PTLD), intraluminal calculus or blood clot, and ureteral fibrosis are causes of obstruction [6].
Urine Leaks
Most urine leaks are evident in the immediate postoperative period and are caused by faulty anastomosis or ureteral necrosis resulting from impaired blood supply. Late leaks are associated with ureteral graft rejection.
Urine leak is suspected if a peritransplant fluid collection shows an increase in volume or if a new fluid collection occurs. Radionuclide renography is then indicated to confirm the leak.
Pancreas Transplants
The primary indication for pancreas transplantation is to provide an intrinsic source of insulin for patients with severe insulin-dependent diabetes mellitus. Because many of these patients also have diabetic nephropathy, pancreas transplant is commonly (~90% of pancreas transplants) combined with simultaneous renal transplant. Ideal candidates are patients with type I diabetes who are younger than age 45 and have little or no atherosclerotic disease. Most pancreatic transplants are whole organ transplants harvested intact from cadavers, which may also be the source of the renal transplant. Live donor grafts are always segmental, have a smaller mass of beta cells and have a higher risk of thrombosis.
Surgical Technique
Traditional whole organ pancreatic transplantation involves arterial anastomosis of the celiac axis/splenic artery with the external iliac artery, systemic venous drainage with anastomosis of the splenic/portal vein to the external iliac vein, and drainage of pancreatic exocrine secretions into the bladder through an attached segment of duodenum [12]. Vascular anastomoses are usually made end-to-side. The donor splenic and superior mesenteric arteries may be joined in a Y-graft and then anastomosed to the iliac artery. The transplanted pancreas is usually intraperitoneal in location. This procedure has been associated with a variety of urologic complications, reflux graft pancreatitis, and hematuria. Systemic venous drainage is associated with hyperinsulinemia and peripheral insulin resistance. Usually the pancreas is placed in one iliac fossa and the kidney is placed in the other iliac fossa.
To combat complications of the traditional procedure, another technique with venous drainage to the portal system and exocrine drainage to the intestinal tract is used at some institutions [13]. The pancreas is placed in the lower abdomen surrounded by bowel and mesentery.
Pancreas Transplant Scan Protocol
The goals of imaging are to detect and characterize complications, to guide aspiration of fluid collections, and to guide biopsy of the transplanted pancreas. Sonography is indicated for routine follow-up and whenever transplant dysfunction or complication is suspected. All US examinations should include Doppler evaluation of the parenchyma and its vascular connections. Knowledge of the vascular anastomoses of the specific transplant examined is most helpful in its evaluation.
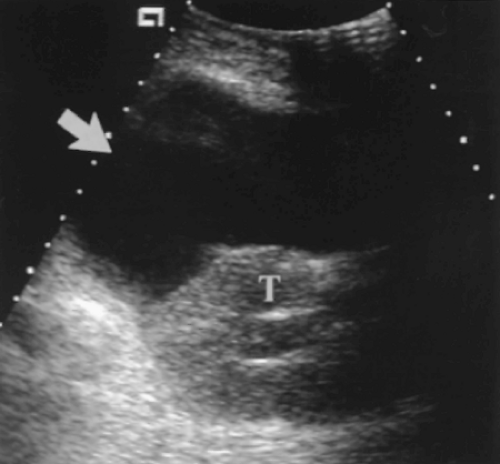
The pancreas is evaluated for parenchymal echogenicity, location, and orientation.
Measurement of the size of the graft should be made to establish a baseline for detection of enlargement that is associated with acute rejection and acute pancreatitis.
A careful search for peripancreatic fluid collections is undertaken.
Arterial and venous flow is carefully evaluated with color flow and spectral Doppler.
Normal Pancreas Transplant
In the immediate postoperative period, the transplanted pancreas commonly shows focal abnormalities related to handling and surgical trauma. The graft may appear focally or diffusely hypoechoic, even though the pancreas is functioning normally. The graft appearance should be normal within 2-4 weeks provided no complications intervene.
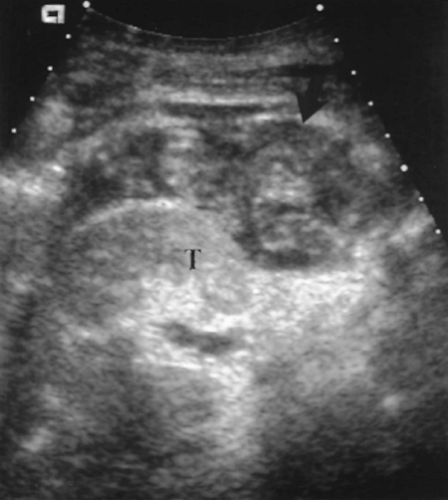
Normal pancreatic parenchyma shows homogeneous mid-level echogenicity slightly greater than muscle (Fig. 14.8).
Doppler confirms venous and low resistance arterial flow.
Complications of Pancreas Transplantation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree