Fig. 13.1
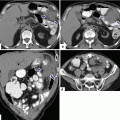
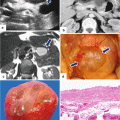
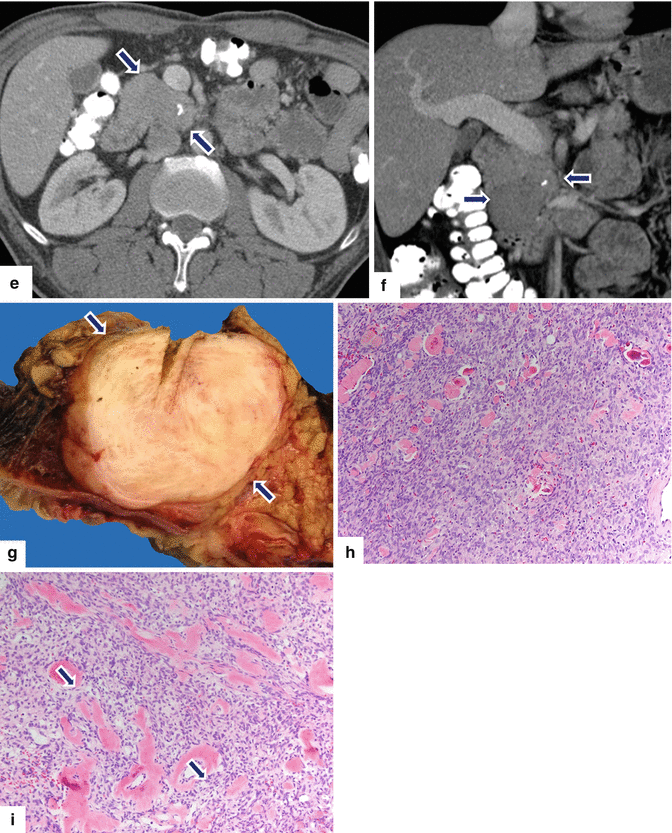
Solitary fibrous tumor of the pancreas. A 45-year-old male patient with history of epigastric discomfort. Ultrasound transverse (a, b) images show a hypoechoic mass in the pancreatic head with strong sound attenuation (arrows). CECT axial (c–e) and coronal (f) images show an isodense solid mass with a focal calcification involving the pancreatic head and uncinate process (arrows). Note the absence of dilatation of the pancreatic duct or common bile duct associated with this mass. The patient underwent a Whipple procedure. Photograph of the bivalved gross specimen (g) shows a firm, sclerotic, white, tan mass. Histological sections (h, i) (H&E, 10×, 20×) show spindle cells (arrows) with collagen bands and hyalinized blood vessels
Ultrasound (US): hypoechoic, hypovascular mass. This mass can be associated with significant sound attenuation due to its fibrous nature.
Computed tomography (CT): well-demarcated mass with progressive contrast enhancement.
Magnetic resonance (MR), T1WI: low signal intensity mass.
T2WI: heterogeneous signal intensity with spotty hyperintense foci and capsule-like hypointense rim.
T1WI with gadolinium: progressive enhancement.
13.2.6 Differential Diagnosis
Neuroendocrine tumor, acinar cell carcinoma, solid pseudopapillary tumor, pancreatic lymphoma, and pancreatic hemangioendothelioma.
13.2.7 Treatment
SFTs have malignant potential; consequently, the treatment of choice is complete resection.
13.3 Inflammatory Myofibroblastic Tumor of the Pancreas (IMT) (Pseudo-tumor) (Fig. 13.2)
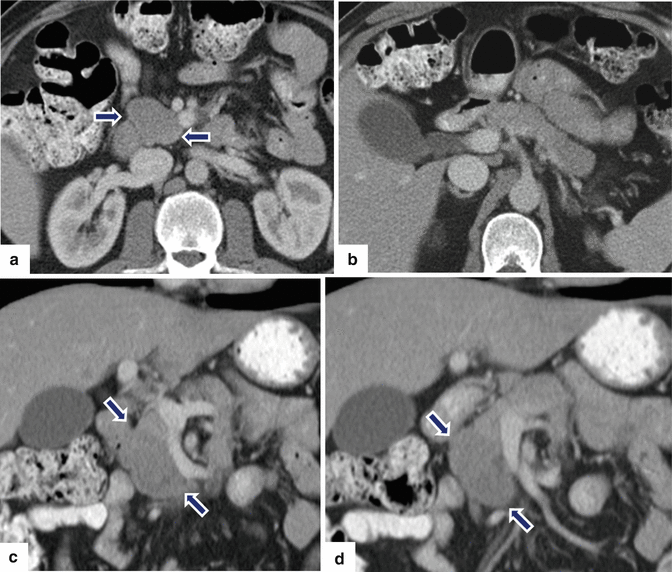
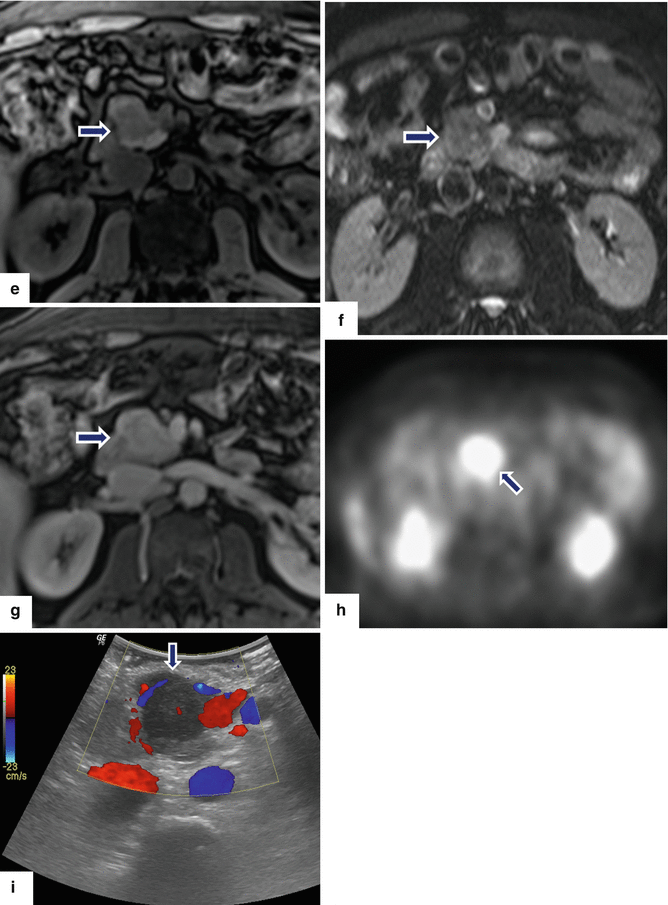
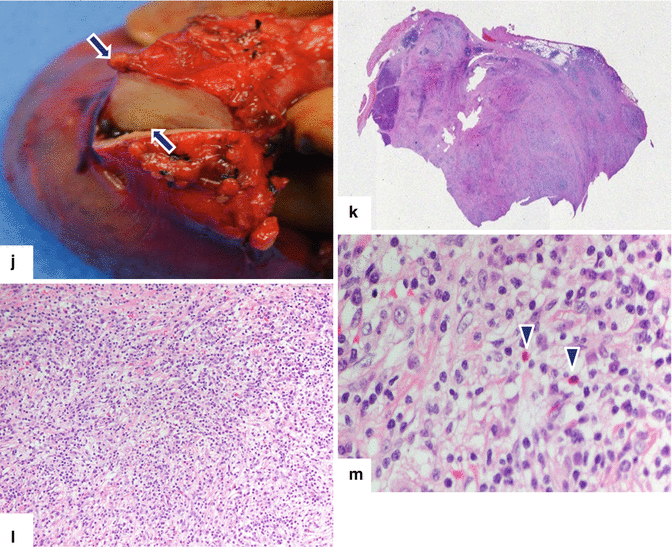
Fig. 13.2
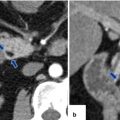
Inflammatory myofibroblastic tumor of the pancreas (pseudo-tumor). A 43-year-old male patient with history of diabetes, hypertension, and right upper quadrant pain. CECT axial (a, b) and coronal (c, d) images demonstrate a homogeneous, hypodense mass in the pancreatic head (arrows).On fat-suppressed gradient echo T1-weighted axial (e) imaging, this mass has low signal intensity (arrow). On fat-suppressed T2-weighted (f) imaging, this mass has heterogeneous signal intensity (arrow). On contrast-enhanced fat-suppressed gradient echo T1-weighted (g) imaging, this mass demonstrates diffuse, homogeneous enhancement (arrow). On PET scan (h), this mass shows avid FDG uptake (arrow). IOUS transverse (i) image shows a hypoechoic, hypovascular mass. The patient underwent a pyloric-sparing Whipple procedure.Photograph of the bivalved specimen (j) demonstrates a firm, homogenous, pale yellow mass. Histological sections demonstrate distortion of the pancreatic architecture, fibrosis (k) (H&E, 2×), and myofibroblasts admixed with inflammatory infiltrate, including eosinophils (arrowheads), plasma cells, and lymphocytes (l, m) (H&E, 10×, 60×)
Inflammatory myofibroblastic tumor (IMT) occurs in various organs, the lung being the most common anatomic site
Occurs most frequently in the first two decades of life
Etiology unknown
Pancreatic IMTs are extremely rare
Median size: 1.5–13 cm
Most commonly located in the pancreatic head
13.3.1 Macroscopic Appearance
Well-circumscribed or multinodular, non-encapsulated, white, firm, mass with a whorled fleshy cut surface
13.3.2 Microscopic Appearance
This tumor is composed of spindle-shaped myofibroblasts or fibroblasts accompanied by a mixed inflammatory infiltrate of eosinophils, plasma cells, and lymphocytes.
Immunohistochemistry: positive for smooth muscle actin (SMA) and ALK1 (40 % of the cases) and negative for keratin, CD34, and caldesmon.
13.3.3 Clinical Evaluation
Generally asymptomatic, incidental finding on imaging
May be associated with abdominal pain, weight loss, jaundice, anorexia, anemia, nausea, vomiting, fever, and malaise
13.3.4 Laboratory
Nonspecific
13.3.5 Imaging
Nonspecific findings
US: hypoechoic mass with well- or ill-defined margins.
Plain CT: low attenuation or isodense mass, homogeneous or heterogeneous.
CECT: poor enhancement in arterial phase; mild enhancement in portal phase.
MR: T1WI/T2WI low signal intensity mass.
Contrast-enhanced MR T1WI: mild enhancement in the portal phase.
13.3.6 Differential Diagnosis
Pancreatic adenocarcinoma, pancreatic neuroendocrine neoplasm, pancreatic lymphoma, solitary fibrous tumor, and pancreatic metastases
13.3.7 Treatment
Surgical resection
13.4 Pancreatic Lymphoma (PL)
PL is classified as either primary or secondary.
Most common subtype: B-cell non-Hodgkin lymphoma.
Secondary involvement is more common than primary lymphoma.
Secondary lymphoma can be identified in up to 30 % of patients with widespread lymphoma.
Incidence is as high as 5 % in human immunodeficiency virus (HIV) patients.
Primary is much less common and accounts for less than 2 % of extranodal non-Hodgkin lymphoma.
Sex: slight male predominance.
Age: affects patients in the 5th or 6th decade of life.
Primary PL
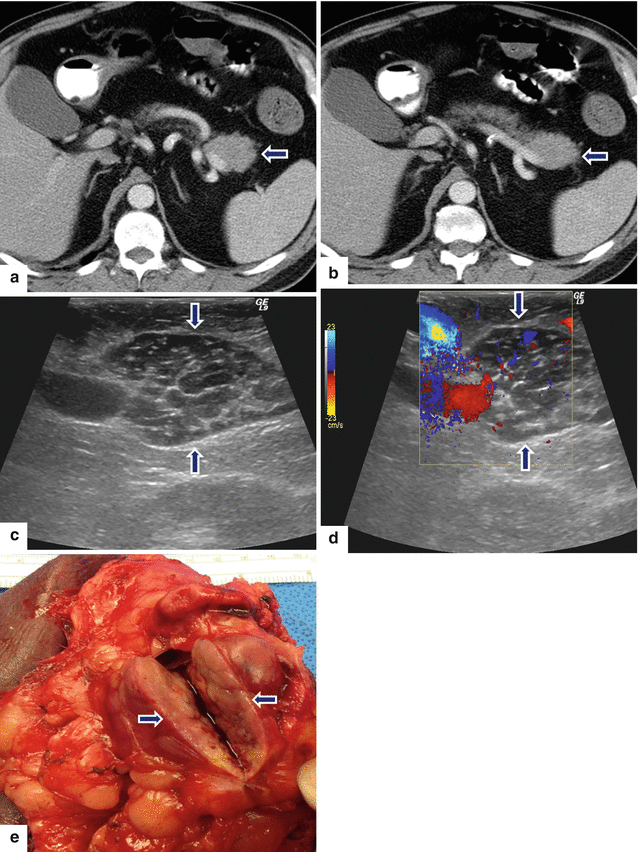
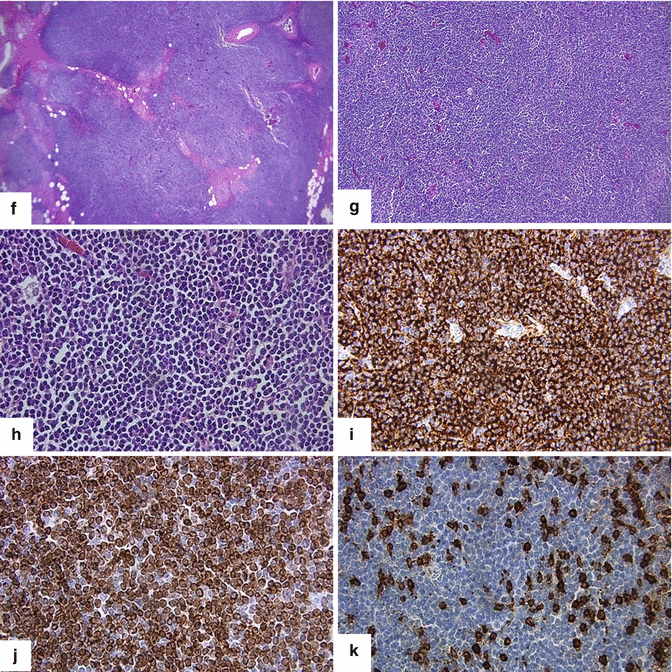
Fig. 13.3
Primary pancreatic lymphoma. A 68-year-old male with history of respiratory symptoms who underwent a chest CT that demonstrated an incidental pancreatic lesion. CECT axial (a, b) images show a homogeneous mass in the pancreatic tail (arrows). IOUS gray scale and color Doppler transverse (c, d) images show a well-circumscribed avascular, hypoechoic mass with a trabeculated pattern (arrows). The patient underwent a distal pancreatectomy and splenectomy. Photograph of the bivalved mass (e) shows a fleshy, pale gray, soft tissue mass (arrows).Histological sections show a monotonous lymphocytic cell population replacing the pancreatic parenchyma (f, g); this neoplasm is composed of small lymphocytes (h). By immunohistochemistry, the tumor cells are positive for CD20 (i) and BCL2 (j) and negative for CD3 (k); consistent with a follicular B-cell lymphoma (H&E, 4×, 10×, 60×)
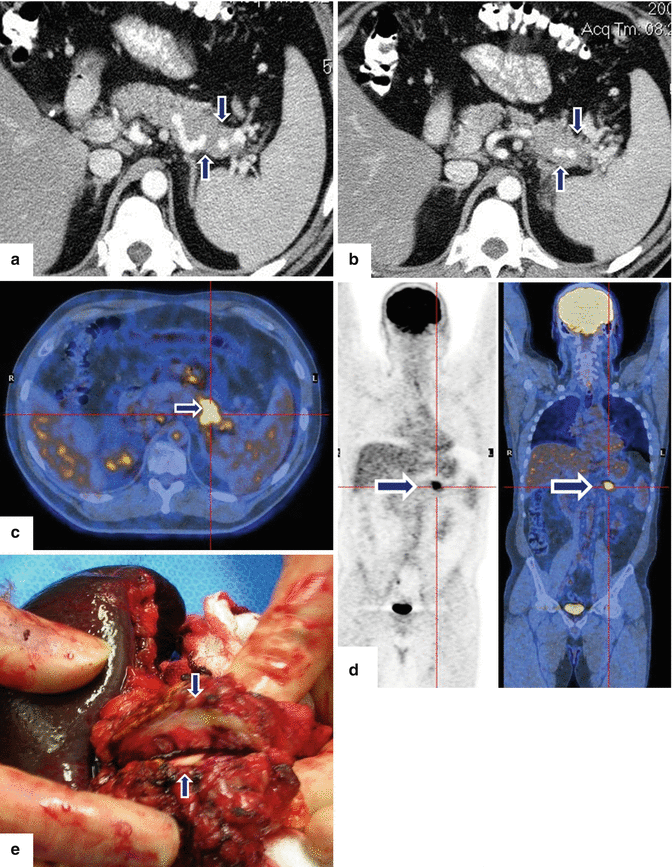
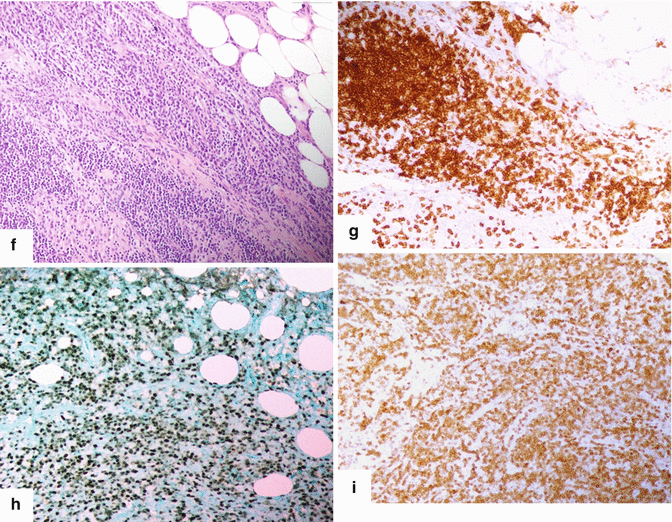
Fig. 13.4
Primary pancreatic lymphoma. A 49-year-old male with an incidentally discovered pancreatic mass on abdominal CT, to rule out renal stones. CECT axial (a, b) images show an infiltrative, poorly defined, hypodense mass involving the pancreatic tail (arrows). PET/CT axial (c) and coronal (d) images show avid FDG uptake by this mass (arrows). The patient underwent a distal pancreatectomy and splenectomy. Photograph of the bivalved mass (e) shows a white, pale, infiltrative mass (arrows).Histological sections show a lymphocytic cell population infiltrating adjacent fat (f). These lymphocytes are positive for CD20 (g), MUM-1 (h), and BCL-2 (i), (H&E, 10×), (immunoperoxidase, 10×). Findings consistent with a B-cell lymphoma
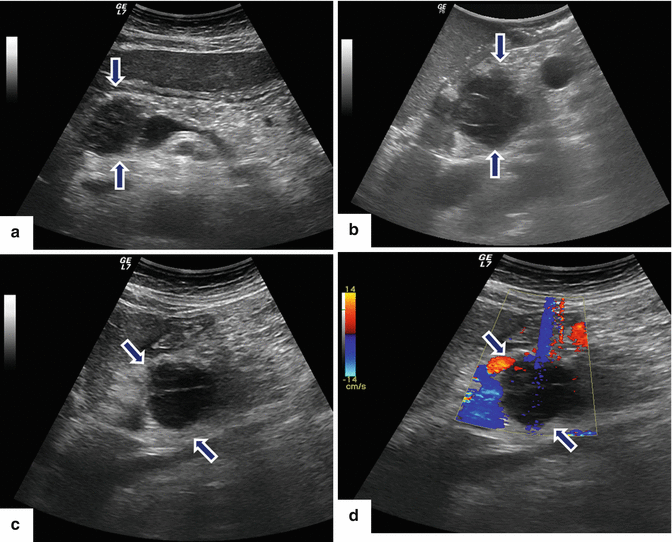
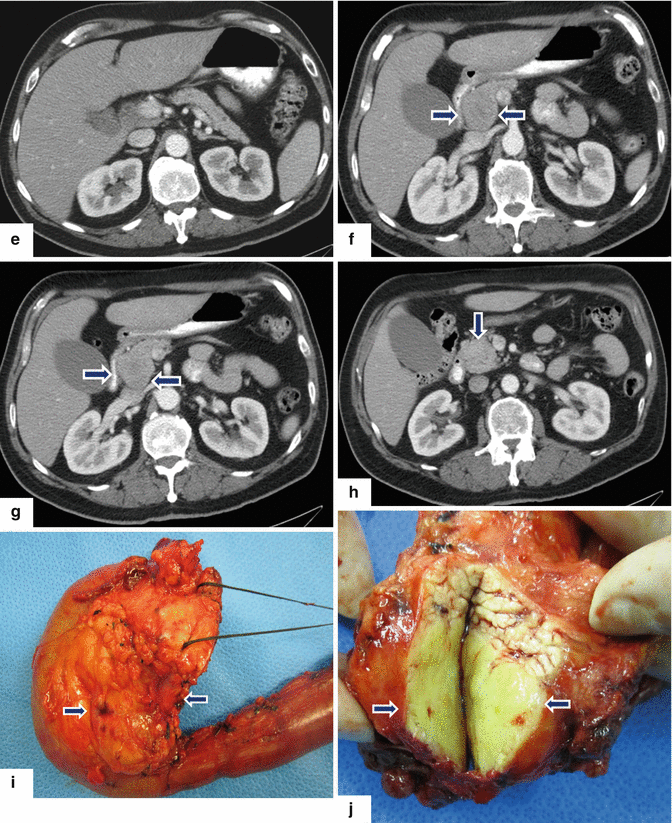
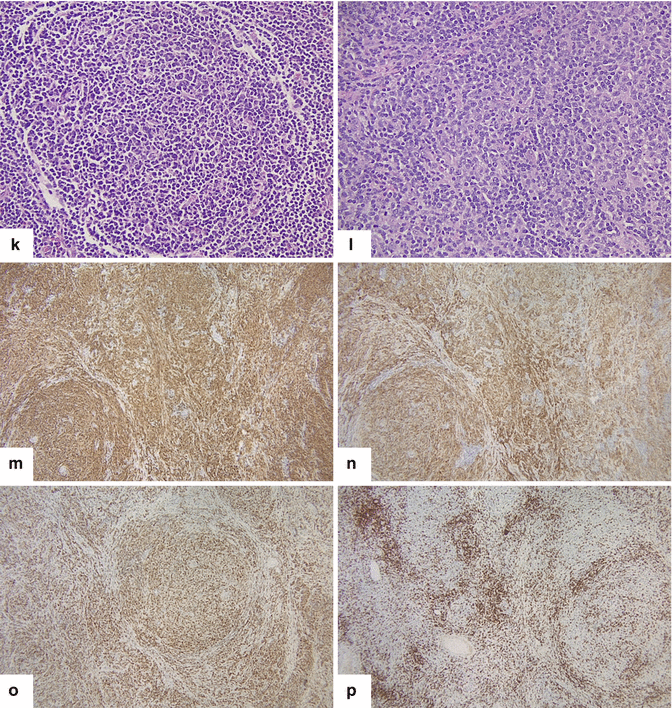
Fig. 13.5
Primary pancreatic lymphoma. A 52-year-old male diabetic with history of significant weight loss and diarrhea four or five episodes daily. US transverse (a, b) and sagittal (c, d) images show a well-defined, avascular, hypoechoic mass with a reticular pattern in the pancreatic head (arrows).CECT axial (e–i) images confirm the presence of a heterogeneous mass in the pancreatic head (arrows). Note the absence of the dilatation of the common bile duct or the pancreatic duct. This mass was biopsied under EUS guidance. The results of the biopsy were inconclusive. The patient underwent a pyloric-sparing Whipple procedure. Photograph of the surgical specimen (i) shows a mass with smooth serosal surface. Photograph of the bivalved mass (j) shows a soft, pale yellow, well-circumscribed mass (arrows).Histological sections show a neoplasm with nodular (k) diffuse infiltrative pattern (l) (H&E, 60×) composed of B lymphocytes that are positive for CD20 (m), CD10 (n), and BCL-2 (o) and negative for CD5 (p) (immunostains, 10x). Findings consistent with follicular B-cell lymphoma
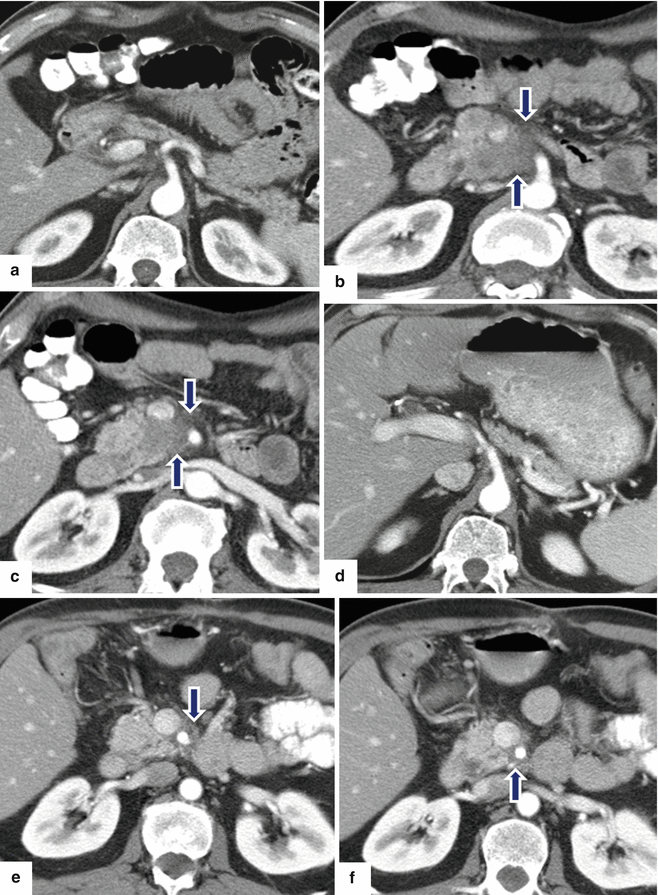
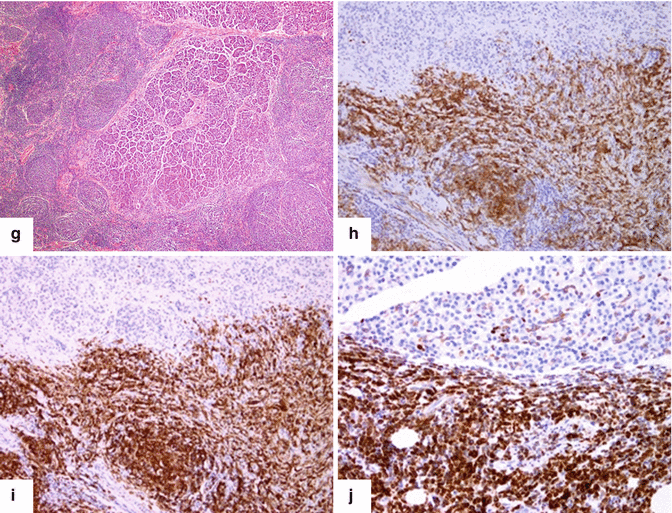
Fig 13.6
Primary pancreatic lymphoma. A 47-year-old male with history of chronic abdominal pain found to have a pancreatic mass. CECT axial (a–c) images show an infiltrative mass of low attenuation involving the pancreatic head and peripancreatic fat (arrows), partially encasing the SMV and SMA. This mass was biopsied under EUS guidance. The results of this biopsy were inconclusive. The patient then underwent an open surgical biopsy which revealed a pancreatic lymphoma. Patient was treated with 6 cycles of R-CHOP chemotherapy and radiation. Surveillance abdominal CECT 9 months later (d–f) shows complete resolution of the pancreatic mass with minimal residual fibrotic tissue (arrows).Histological sections show a follicular B-cell lymphoma infiltrating the pancreatic parenchyma (g), positive for CD10 (h), CD20 (i), and BCL-2 (j) (H&E, 10×) (Immunostains, 20×)
Involvement is confined to the pancreas and immediately adjacent lymph nodes.
Secondary PL:
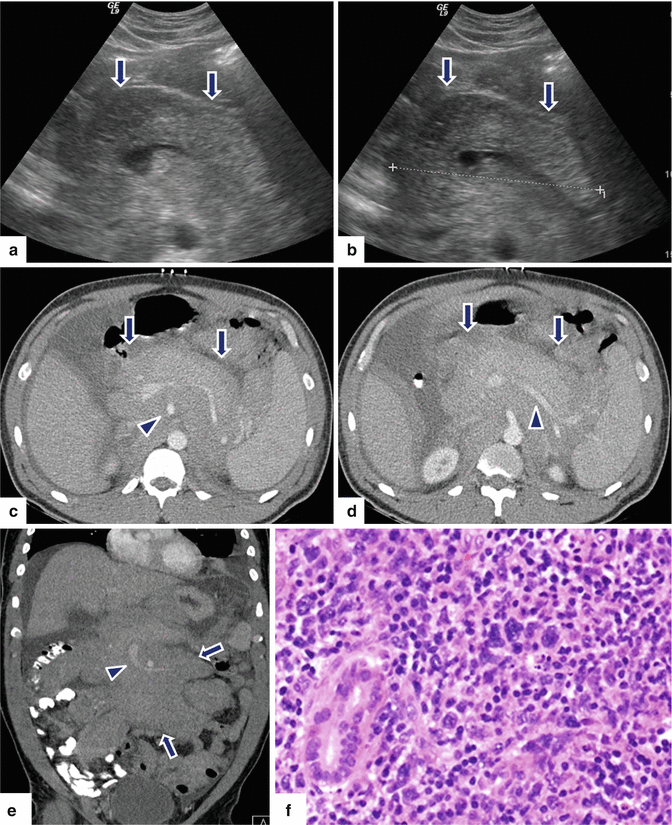
Fig. 13.7
Secondary pancreatic lymphoma. A 46-year-old female with history of abdominal tenderness, early satiety, weight loss, and night sweats. Ultrasound transverse (a, b) images demonstrate diffuse enlargement of the pancreas. Note the heterogeneous echogenicity of this gland (arrows). CECT axial (c, d) and coronal (e) images demonstrate a diffuse, hypodense, homogeneous, retroperitoneal mass infiltrating the retroperitoneal fat and pancreas and encasing the aorta, superior mesenteric artery (SMA), and splenic and superior mesenteric veins (arrowheads). Note the presence of a moderate amount of ascites and the presence of mild splenomegaly. This retroperitoneal mass was biopsied percutaneously with CT guidance. The biopsy results show a B-cell lymphoma. The patient was treated with R-CHOP chemotherapy resulting in complete remission. Histological section (f) (H&E, 60×) demonstrates a diffuse, large B-cell lymphoma infiltrating the pancreatic parenchyma
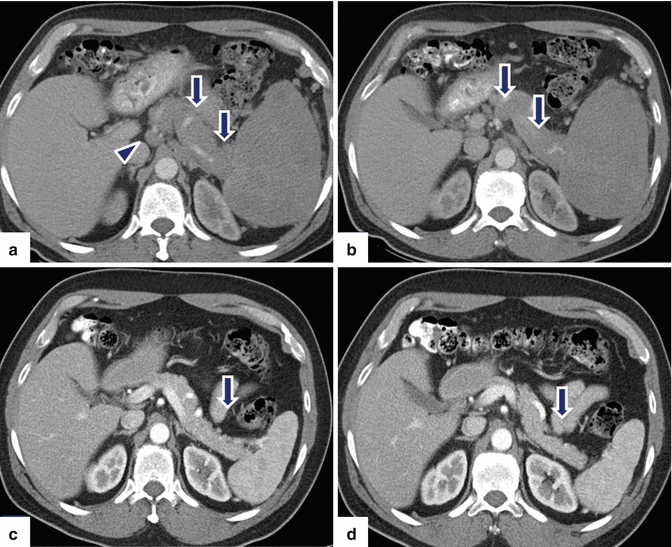
Fig. 13.8
Secondary pancreatic lymphoma. A 53-year-old male with history of left upper quadrant abdominal pain, fever, chills, and night sweats. On physical examination, the patient had enlarged cervical lymph nodes and marked splenomegaly. CECT axial (a, b) images reveal diffuse enlargement of the pancreas (arrows) with lack of the normal lobulations and splenomegaly. Note the presence of a large pericaval node (arrowhead). A percutaneous biopsy of a peripheral node in the neck revealed diffuse large B-cell lymphoma. Patient was treated with chemotherapy. CECT (c, d) performed 3 months later reveal dramatic response to the treatment. Note the normal appearance of the pancreas (arrows) and spleen and the resolution of the enlarged pericaval node
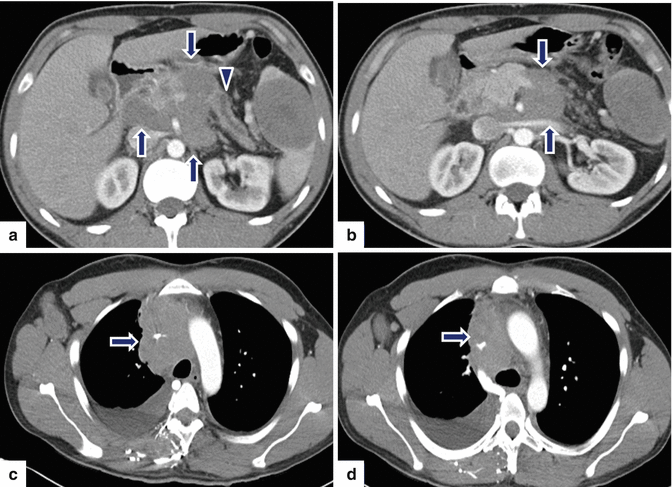
Fig. 13.9
Secondary pancreatic lymphoma. A 31-year-old male with history of chest pain and shortness of breath. CECT axial images of the abdomen (a, b) demonstrate a large, low attenuation, lobulated mass involving the retroperitoneum and infiltrating the pancreatic body (arrows) and producing obstruction of the pancreatic duct (arrowhead). Note the presence of a large round mass of low attenuation in the spleen. CECT axial images of the chest (c–d) demonstrate mediastinal adenopathy (arrows) encasing the superior vena cava, and a right pleural effusion. Final pathology: diffuse large B-cell lymphoma
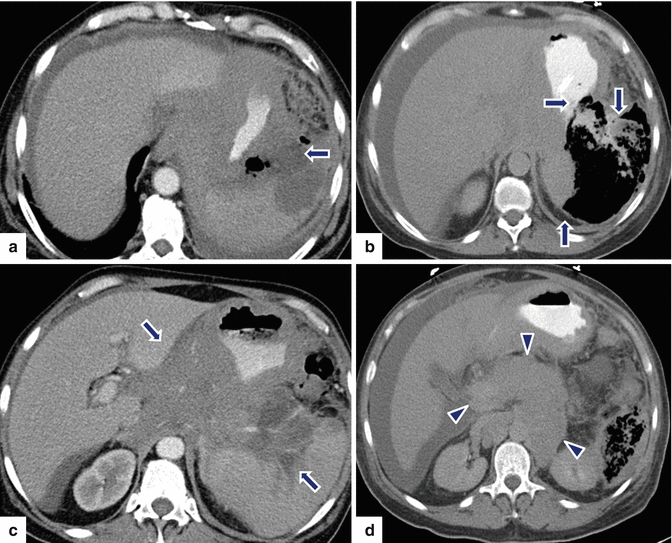
Fig. 13.10
Secondary pancreatic lymphoma. A 63-year-old male with history of AIDS admitted to the hospital with septic shock. CECT axial (a–d) images show a large complex collection mixed with air and fluid in the left upper quadrant (arrows). Note the poorly defined retroperitoneal mass infiltrating the stomach and encasing the celiac artery and its branches, and diffuse enlargement of the pancreas (d) (arrowheads), and the presence of intraperitoneal fluid. The patient was taken to the OR for an exploratory laparotomy. A gastric perforation was noted which was repaired with a Graham patch. Final pathology: diffuse large B-cell lymphoma
Extra pancreatic lymphoma directly extending or infiltrating into the adjacent pancreatic parenchyma.
Usually invasion of the pancreas occurs by contiguous retroperitoneal adenopathy or gastric lymphomas.
Associated lymphadenopathy elsewhere in the abdomen is also common.
13.4.1 Macroscopic Appearance
White, poorly marginated mass with fleshy appearance at cut surface
13.4.2 Microscopic Appearance
Histologic sub-types: follicular lymphoma, lymphoma of mucosa-associated lymphoid tissue, diffuse large B-cell lymphoma, and T-cell lymphoma
13.4.3 Clinical Presentation
Clinical symptoms are nonspecific.
Most common symptoms and signs include abdominal pain, palpable mass, and weight loss. Classic symptoms of NHL (fever, chills, and night sweats) are rare in primary lymphoma.
13.4.4 Laboratory Evaluation
Nonspecific
Lactate dehydrogenase (LDH) may be elevated.
13.4.5 Imaging
Primary lymphoma (PL)
US: round, well-defined, homogeneous, hypoechoic avascular mass with or without posterior attenuation of sound. These masses may exhibit a trabecular/reticular pattern.
Plain CT: isodense, well-circumscribed or infiltrative mass.
CECT: mass associated with mild homogeneous enhancement.
MR: T1WI: low signal intensity mass. T2WI: intermediate homogeneous or heterogeneous signal intensity.Contrast-enhanced T1WI: mild enhancement portal phase.
PET/CT: avid FDG uptake by the pancreatic mass.
Secondary Lymphoma (SL)
Occurs due to the invasion of the pancreas by a bulky or infiltrative mass arising from the retroperitoneum, stomach, or both.
Diffuse or focal pancreatic enlargement.
US: hypoechoic or heterogeneous echogenicity of the pancreatic parenchyma.
CT: mild homogeneous enhancement, ill-defined margins.
MR: T1WI: diffuse low signal intensity. T2WI: heterogeneous signal intensity. Contrast-enhanced T1WI: mild homogeneous enhancement.
13.4.6 Differential Diagnosis
PL: pancreatic adenocarcinoma, focal autoimmune pancreatitis, pancreatic pseudocyst, myofibroblastic tumor, solitary fibrous tumor, and pancreatic metastases
SL: autoimmune pancreatitis, acute pancreatitis, diffuse pancreatic adenocarcinoma, or diffuse pancreatic metastases
13.4.7 Treatment
Chemotherapy is the treatment of choice. Most common regimen includes cyclophosphamide, doxorubicin, hydrochloride, vincristine, and prednisone.
Practical Pearls
Primary lymphoma may have a very similar appearance by imaging to a pancreatic adenocarcinoma. Therefore, it is always important to verify the diagnosis of a pancreatic mass with percutaneous, endoscopic, or laparoscopic core biopsy to select the appropriate therapy.
Enlarged peripancreatic metastatic nodes from the colon, breast, and gastric carcinomas infiltrating the pancreas may mimic a secondary lymphoma.
Vascular stenosis or occlusion is rarely seen in pancreatic lymphoma,
Lymphadenopathy, located below the level of the renal veins, is more common in patients with pancreatic lymphoma than in patients with pancreatic adenocarcinoma.
Elevation of serum amylase or lipase may be detected in secondary pancreatic lymphomas.
13.5 Acinar Cell Carcinoma (ACC) (Figs. 13.11–13.14)
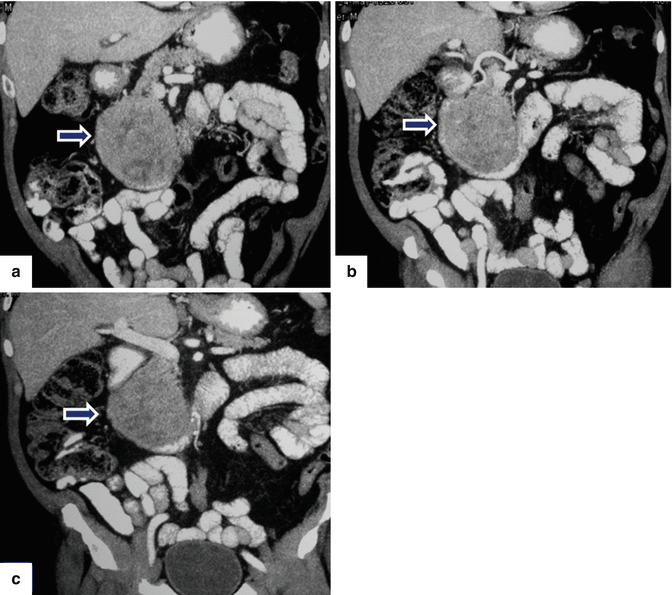
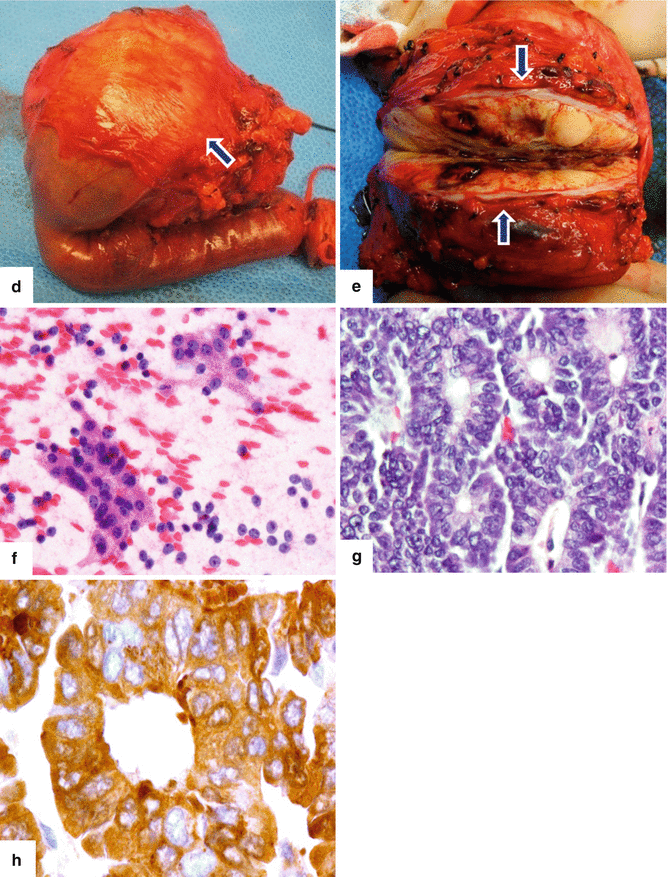
Fig. 13.11
Acinar cell carcinoma of the pancreas. An 85-year-old male with history of malaise, anorexia, and weight loss of 10–15 lbs, over 2–3 months. He was admitted to the hospital with UGI bleeding. CECT coronal (a–c) images show a large, heterogeneous, well-circumscribed mass involving the pancreatic head (arrows) and abutting the duodenal loop.The patient underwent a Whipple procedure. Photograph of the resected specimen (d) shows a large mass with smooth capsular surface. Photograph of the bivalved specimen (e) shows a heterogeneous, firm, pale yellow mass with hemorrhagic foci (arrows). Cytology sample (f) shows two aggregates of cells with abundant eosinophilic granular cytoplasm and nuclei with nucleoli. Note the naked nuclei in the background. Histologically, the cells show acinar formation, granular cytoplasm and prominent nucleoli (g) (H&E, 40×). By immunohistochemistry, the tumor cells are positive for trypsin (h) (Immunostain, 100×). Findings are consistent with acinar cell carcinoma
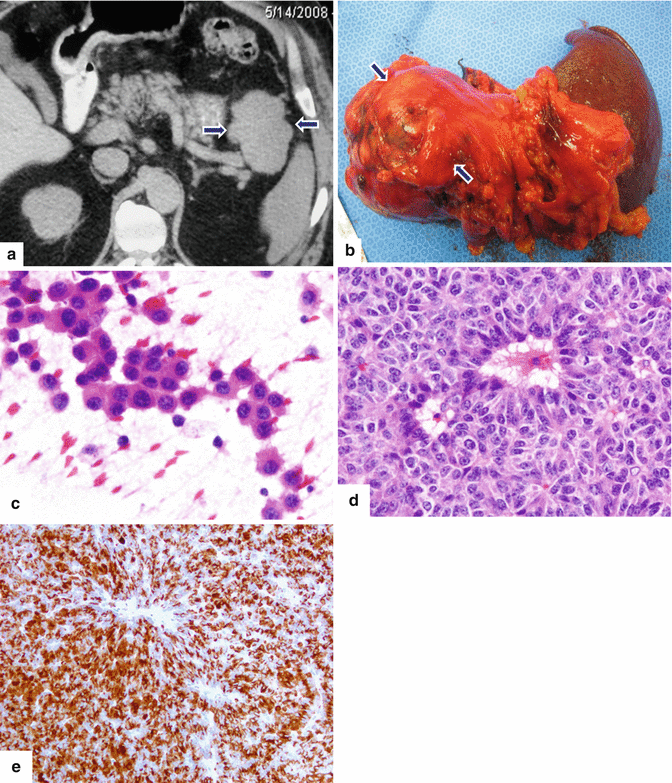
Fig. 13.12
Acinar cell carcinoma of the pancreas. A 69-year-old male admitted to the hospital for epigastric pain. Plain CT axial (a) image reveals a large, homogeneous, lobulated mass involving the pancreatic tail (arrows). The patient underwent a distal pancreatectomy and splenectomy. Photograph (b) of the gross specimen reveals a large, bulky, lobulated mass. Cytology of this moderately differentiated acinar cell carcinoma demonstrates loosely cohesive tumor cells with mild anisonucleosis, eosinophilic cytoplasm and prominent nucleoli (c). Histological sections (d) demonstrate tumor cells with focal acinar formation, granular cytoplasm, prominent nucleoli and a positive trypsin immunostain (e) (H&E, 40×) (Immunoperoxidase, 20×)
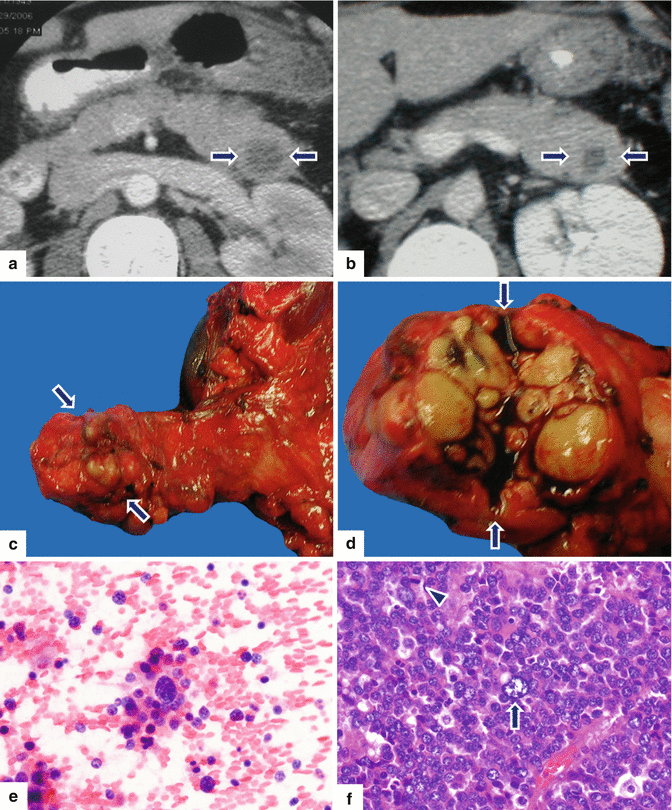
Fig. 13.13
Acinar cell carcinoma of the pancreas. A 56-year-old female with history of recurrent pancreatitis. CECT axial (a, b) images demonstrate a round, low attenuation mass in the pancreatic tail (arrows). The patient underwent a distal pancreatectomy and splenectomy (c). Photograph of the gross specimen (d) demonstrates a multinodular, pale, yellow, mass (arrows). Cytology of this poorly differentiated acinar cell carcinoma (e) demonstrates isolated tumor cells with scant cytoplasm and nuclear pleomorphism. Histological section (f) demonstrate tumor cells with marked pleomorphism, prominent nucleoli, few nuclei with open chromatin (arrow), and increased mitotic figures (f) (arrowhead) (H&E, 40×)
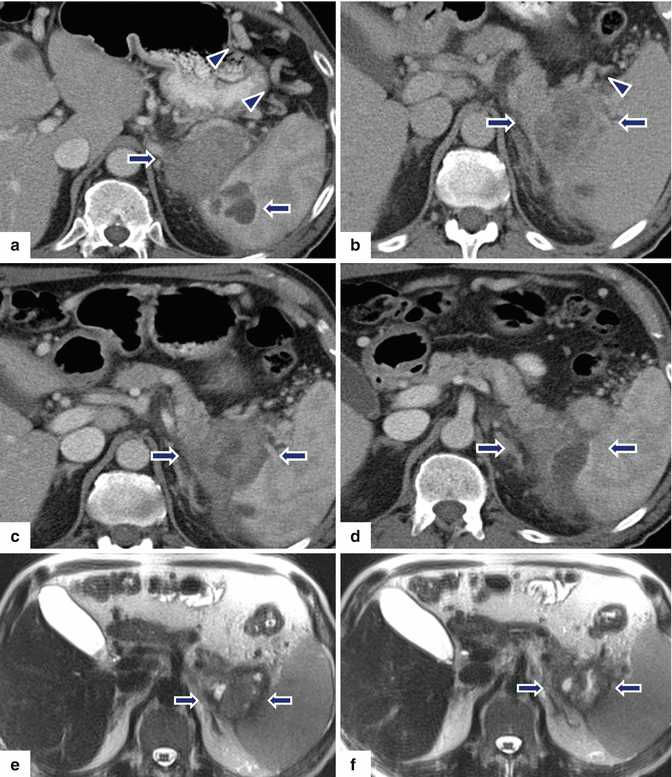
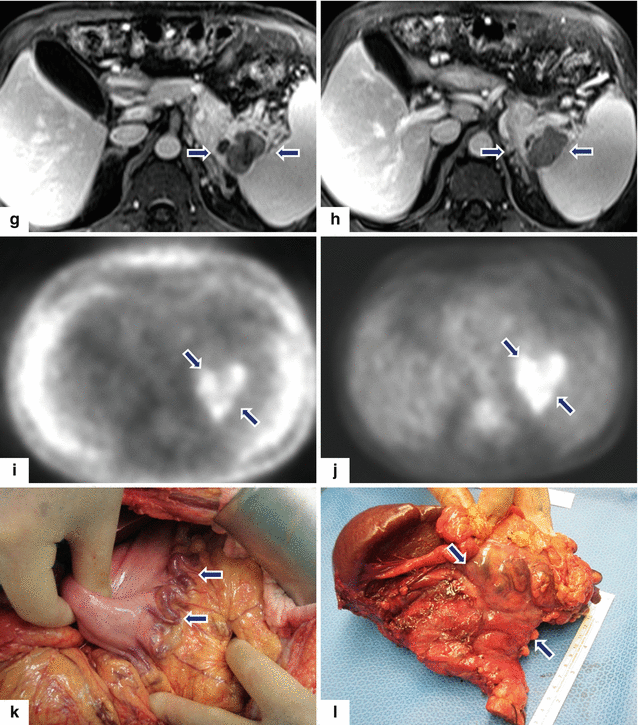
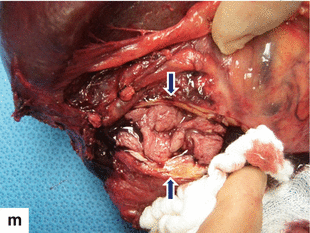
Fig. 13.14
Acinar cell carcinoma of the pancreas. A 67-year-old male with history of prostatic and skin carcinoma, who presented with an episode of acute pancreatitis. CECT axial (a–d) images show a large, heterogeneous mass (arrows) with areas of the necrosis and poorly defined margins involving the pancreatic tail and extending into the spleen. Note the lack of identification of the splenic vein and the presence of prominent, short gastric veins (arrowheads), findings suggestive of splenic vein thrombosis. Single-shot fast spin echo T2-weighted axial (e, f) and contrast-enhanced fat-suppressed gradient echo T1-weighted (g, h) images confirm the presence of a complex mass in the pancreatic tail and extending into the spleen. PET scan axial (i, j) images show a large FDG avid pancreatic mass (arrows). Intraoperative photograph (k) confirms the presence of prominent, short gastric veins (arrows). The patient underwent a distal pancreatectomy and splenectomy. Photograph of the gross specimen (l) shows a large, lobulated, bulky mass (arrows). Photograph of the bivalved mass (m) shows a pink, multinodular mass (arrows)
Account for 1 % of primary pancreatic neoplasms.
These tumors typically secrete pancreatic enzymes (trypsin, chymotrypsin, amylase, and lipase).
Most commonly occurs in the fifth to seventh decade of life.
More common in males.
May occur along any part of the pancreas with the head being most common.
Typical mutations seen with pancreatic adenocarcinoma are not common with ACC.
Aggressive biological behavior with overall survival only slightly better than pancreatic adenocarcinoma.
Metastatic disease is seen in up to 50 % of patients at presentation, most commonly in the liver and lymph nodes.
Cell of origin: acinar cells
13.5.1 Macroscopic Appearance
Well-circumscribed, partly encapsulated, pink to tan color, heterogeneous or homogeneous, fleshy mass with fibrous septa, hemorrhage, or necrosis
13.5.2 Microscopic Appearance
Atypical round to oval nuclei with prominent nucleoli
Extremely cellular, with medium-sized polygonal cells
Eosinophilic cytoplasm due to zymogen granules that are acid-Schiff positive and diastase resistant
Immunohistohemical stains: positive for trypsin, pancreatic amylase, and lipase. Negative for neuroendocrine markers (synaptophysin, chromogranin)
13.5.3 Clinical Presentation
Usually nonspecific abdominal pain, nausea, emesis, weight loss, and palpable abdominal mass
Lipase hypersecretion syndrome is an associated paraneoplastic syndrome, characterized by subcutaneous nodules, eosinophilia, and arthralgias secondary to hypersecretion of lipase, and occurs in approximately 15 % of the patients.
13.5.4 Laboratory Evaluation
Nonspecific
Patients may have elevated serum lipase and/or amylase, peripheral eosinophilia, and markedly elevated serum alpha-fetoprotein level.
13.5.5 Imaging
Nonspecific
US: hypoechoic and homogeneous or heterogeneous masses.
CECT:Small masses: solid, homogeneous.Large masses: heterogeneous with cystic changes or central necrosis. Well-marginated or poorly defined masses. Typically enhances homogeneously but less than the surrounding pancreatic parenchyma. Peripheral calcifications may be identified.
MR: T1W1: low signal intensity mass compared to normal pancreas. Hyperintense areas suggestive of hemorrhage may be seen.
T2WI: slightly hyperintense to normal pancreas.
T1WIFS with gadolinium: homogeneous or heterogeneous enhancement (central necrosis).
13.5.6 Differential Diagnosis
Pancreatic adenocarcinoma, neuroendocrine tumor, solid pseudopapillary tumor of the pancreas, and pancreatic metastases
13.5.7 Treatment
Surgical resection.
Aggressive chemotherapy or radiofrequency ablation is warranted in patients with locally advanced disease or recurrence.
13.6 Granulocytic Sarcoma of the Pancreas (GS) (Fig. 13.15)
Granulocytic sarcoma is an extramedullary collection of myeloblasts.
This tumor is also known as chloroma or myeloblastoma.
Usually arise in the course or after the course of acute myeloid leukemia (AML) and other myeloproliferative disorders.
It can be the first and only manifestation of AML.
The most common cytogenetic abnormality found in leukemic patients with GS is the translocation (8:21) (q22:q22).
Most common sites of involvement are the soft tissue, periosteum, lymph nodes, and skin.
Many organs may be affected.
GS of the pancreas is extremely uncommon.
13.6.1 Macroscopic Appearance
Vary in color: green tint, white, gray, or brown soft tissue.
Multilobulated mass, with fleshy appearing cut surface
13.6.2 Microscopic Appearance
Sheets of immature granulocytes, monocytes, or both involving any extramedullary site.
Mature or immature eosinophils may be present.
Immunohistochemical stains: positive for CD34, myeloperoxidase (MPO), CD117, CD68, and CD33.
13.6.3 Clinical Presentation
Symptoms and signs: epigastric pain and jaundice
13.6.4 Laboratory Evaluation
Serum markers are normal.
13.6.5 Imaging
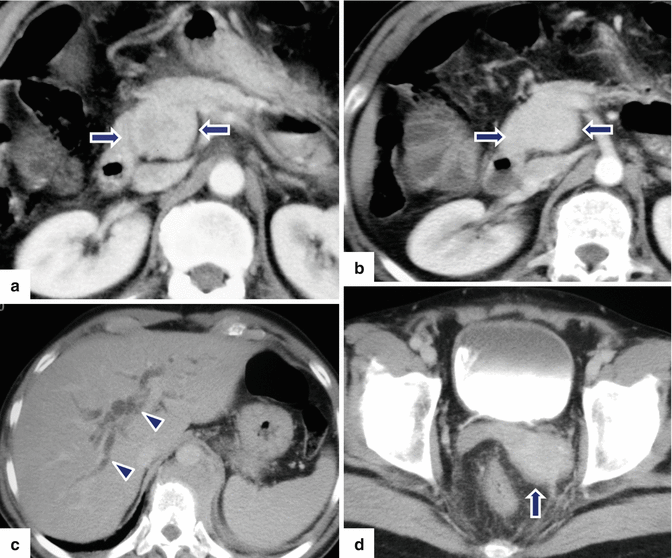
Fig. 13.15
Granulocytic sarcoma of the pancreas. A 64-year-old male with history of acute myeloid leukemia with 70 % of blasts showing complete remission after chemotherapy. Four months later, the patient returned to the hospital with diarrhea, mild abdominal pain, and jaundice lasting a week. CECT axial (a–d) images demonstrate a homogenous mass involving the pancreatic head (a, b) (arrows) associated with dilatation of the biliary system (c) (arrowheads), retrocrural adenopathy, and a mass with the same characteristics in the left seminal vesicle (d) (arrow). Biopsy of the pancreatic mass was performed under CT guidance. The biopsy showed tumor cells that were positive for myeloperoxidase, CD34, and negative for CD20
US: hypoechoic, avascular mass.
CT: well-circumscribed, homogeneous mass with mild enhancement in the portal phase.
MR: T1WI: low signal intensity mass.T2WI: heterogeneous signal intensity.Contrast-enhanced T1WI: homogenous or heterogeneous enhancement.
May be associated with obstruction of the common bile duct, presence of enlarged nodes or involvement of adjacent or distant organs.
13.6.6 Differential Diagnosis
Pancreatic lymphoma, neuroendocrine tumor, pancreatic metastases, and focal autoimmune pancreatitis
13.6.7 Treatment
GS are sensitive to antileukemic chemotherapy, and as such, it is administered as the initial treatment. If chemotherapy is not enough, radiotherapy or surgery can be used.
Practical Pearls
The diagnosis of GS, especially in the pancreas, is challenging and requires the correlation between pathology and immunohistochemical analysis.
Usually the diagnosis is confirmed by biopsy performed with the assistance of endoscopic ultrasound (EUS) or CT.
Tumor resection should be avoided because it may worsen prognosis and survival and may delay the start of chemotherapy. In case of tumor-related obstructive symptoms, surgical bypass may be preferable.
13.7 Pancreatoblastoma (Figs. 13.16–13.17)
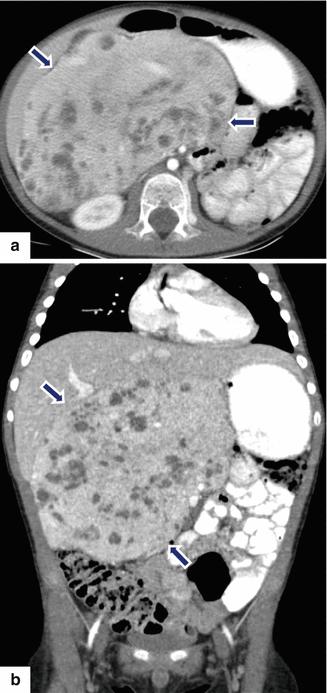
Fig. 13.16
Pancreatoblastoma. A 5-year-old female patient with a palpable abdominal mass. CECT axial (a) and coronal (b) images show a large, well-circumscribed, ovoid mass with heterogeneous attenuation involving the pancreas (arrows) (Courtesy of Ricardo Restrepo, MD)
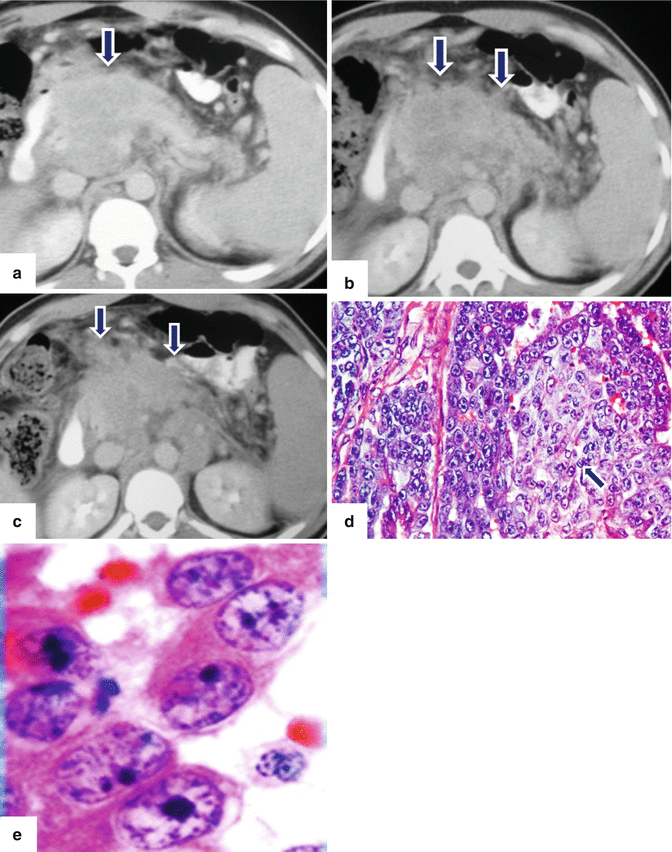
Fig. 13.17
Pancreatoblastoma. A 22-year-old male with history of epigastric pain and weight loss. CECT axial (a–c) images reveal an enhancing, homogenous mass with poorly defined margins involving the head and proximal body of the pancreas (arrows). Note extensive infiltration of the peripancreatic fat by this mass. Histological sections reveal a pancreatoblastoma with poorly differentiated cells, arranged in cords or pseudoacini, and a focus of squamous differentiation (d) (arrow). Higher magnification of the tumor cells (e) reveals eosinophilic, ill-defined granular cytoplasm, and prominent nucleoli (H&E, 60×, 100×)
Rare, malignant pancreatic epithelial neoplasm
Most common pancreatic tumor in children
Variable biological behavior
Better prognosis in children than adults
Metastases occur more often in the liver
More frequent in males
Bimodal Presentation
Children (mean age: 6 years)
Adults (mean age: 40 years)
This tumor may occur sporadically
It may also be associated with genetic syndromes.
Beckwith-Wiedemann syndrome
Familial adenomatous polyposis
13.7.1 Macroscopic Appearance
Well-circumscribed, encapsulated or partially encapsulated, lobulated mass.
The cut surfaces are gray or tan with a soft consistency and focal necrosis.
A cyst variant may be found with Beckwith-Wiedemann syndrome.
13.7.2 Microscopic Appearance
Nests of primitive-appearing cells separated by dense, variably cellular stromal bands.
Composed of acinar cells with focal neuroendocrine or ductal features.
Most characteristic finding: presence of squamoid corpuscles (variable sized foci of squamoid cells).
In children, this tumor is hypercellular and occasionally shows heterogeneous elements such bone or cartilage.
Immunohistochemical stains: positive for pancreatic enzymes (amylase, trypsin, lipase in acinar areas) and P63 and beta-catenin in squamoid corpuscles. Negative for neuroendocrine markers.
13.7.3 Clinical Presentation
Abdominal pain, diarrhea, weight loss, or vomiting
13.7.4 Laboratory Findings
Nonspecific.This tumor may produce alpha-fetoprotein.
13.7.5 Imaging
Most pancreatoblastomas arise from the pancreatic body and/or tail or involve the entire gland.
May infiltrate other abdominal organs, including the spleen, left kidney, left adrenal, and omentum.
Vascular encasement and calcifications may be present.
US: hypoechoic or heterogeneous, avascular mass with well-defined margins.
CT: homogeneous or heterogeneous in attenuation mass with mild heterogeneous enhancement.
MR: T1WI: low signal intensity mass. T2WI: heterogeneous signal intensity. Contrast-enhanced T1WI: heterogeneous enhancement.
13.7.6 Differential Diagnosis
Acinar cell carcinoma, focal autoimmune pancreatitis, neuroblastoma, solid pseudopapillary pancreatic, and neuroendocrine tumors
13.7.7 Treatment
Surgical resection is the only treatment associated with long-term survival.
Chemotherapy and radiation may play a role as a palliative treatment in advanced disease.
13.8 Hemangioendothelioma of the Pancreas (Fig. 13.18)
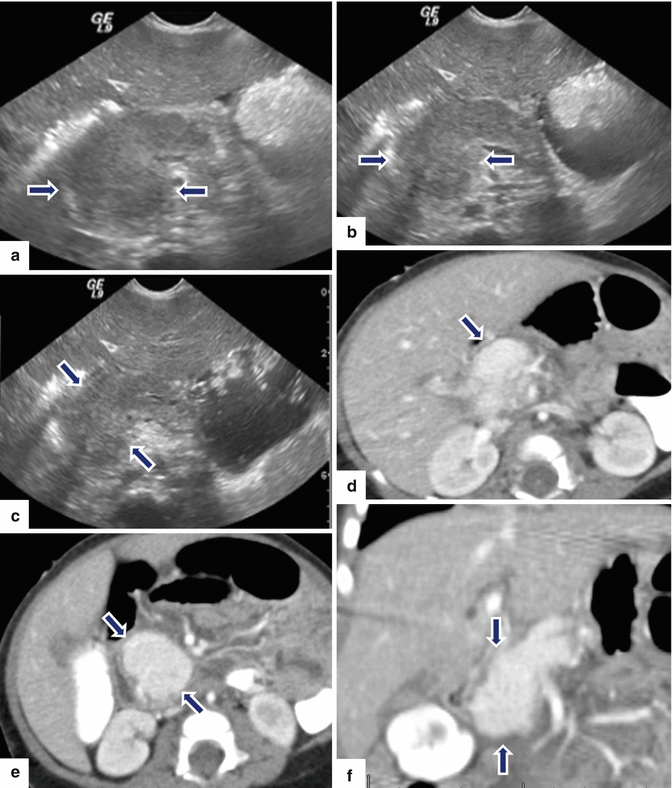
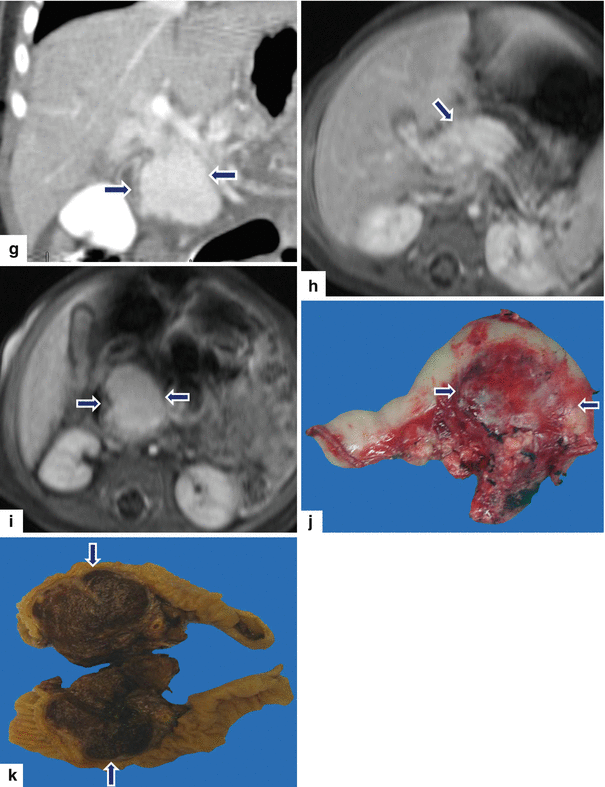
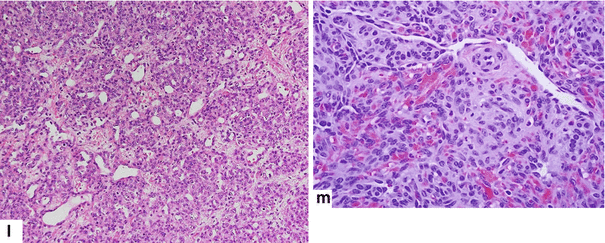
Fig. 13.18
Pancreatic hemangioendothelioma. A 5-month-old male patient referred to our hospital with a suspected diagnosis of biliary atresia. He developed jaundice 9 weeks prior. Ultrasound transverse (a–c) images reveal an isoechoic, well-demarcated mass in the head and neck of the pancreas. CECT axial (d, e) and coronal (f, g) images reveal a hypervascular, homogeneous mass involving the head and neck of the pancreas (arrows). Contrast-enhanced fat-suppressed gradient echo T1-weighted axial (h, i) images confirm the presence of a hypervascular mass in the pancreas (arrows). The patient underwent a Whipple procedure. Photograph of the gross specimen (j) reveals a heterogeneous pancreatic mass with white reddish color and smooth, serosal surface (arrows). Photograph of the fixed bivalved mass (k) reveals a soft, heterogeneous mass (arrows). Histological sections (l, m) reveal a neoplasm composed of small vascular channels of variable size and isolated tumor cells, with few intracytoplasmic vacuoles and scant cytoplasm (H&E, 20×, 40×). (Images courtesy of Gaurav Saigal, MD)
Vascular tumors that may involve numerous organs including the skin, liver, spleen, and salivary glands.
Histologic characteristics and clinical behavior vary between a benign hemangioma and a malignant angiosarcoma.
Often present before 6 months of age.
Tend to grow rapidly and then regress spontaneously over several months.
The pancreas is an extremely rare site of occurrence for this tumor.
Pancreatic head is the most common site.
13.8.1 Macroscopic Appearance
Well-circumscribed firm mass
13.8.2 Microscopic Appearance
Proliferation of capillaries of variable size and neoplastic stromal cells containing cytoplasm with pink to clear foamy PAS + lipid vacuoles .
Immunohistochemical stains: positive for CD31, factor VIII, and FLI1. Negative for keratin.
13.8.3 Clinical Presentation
Obstructive jaundice, hepatomegaly, palpable mass, duodenal obstruction, and intestinal bleeding
13.8.4 Laboratory
Nonspecific
13.8.5 Imaging
US: well-circumscribed, vascular, hypoechoic mass.
CECT: well-circumscribed, homogeneous, hypervascular mass.
MR: T1WI: low signal intensity mass. T2WI: high signal intensity mass.
Contrast-enhanced T1WI: hypervascular mass.
Secondary findings: obstruction of the common bile duct and/or pancreatic duct, venous thrombosis.
13.8.6 Differential Diagnosis
Pancreatoblastoma, neuroendocrine tumor, focal autoimmune pancreatitis, and pancreatic hemangioma
13.8.7 Treatment
Treatment is variable and depends upon the associated biliary obstruction.
Options
Biliary stenting and external drainage pending spontaneous tumor regression
Systemic steroids and interferon
Surgical resection
Practical Pearl
Histopathological confirmation is required in a patient with a suspected HP before a conservative treatment is considered.
13.9 Lipoma of the Pancreas (LP) (Figs. 13.19–13.21)
Lipomas consist of mature adipose cells surrounded by a thin collagen capsule.
Can be found almost anywhere in the body where there is adipose tissue.
Frequently arise from the gastrointestinal tract, when found in the abdominal cavity.
Rarely found in the pancreas.
Most commonly presents as an uneven distribution throughout the pancreas.
Size: 1–15 cm.
13.9.1 Macroscopic Appearance
Well-circumscribed, encapsulated, yellow, soft masses.
13.9.2 Microscopic Appearance
Mature adipocytes with no cellular atypia or pleomorphism. The fat may contain few small capillaries within fibrous strands.
13.9.3 Laboratory Findings
Nonspecific
13.9.4 Clinical Evaluation
Asymptomatic, incidental finding on imaging
13.9.5 Imaging
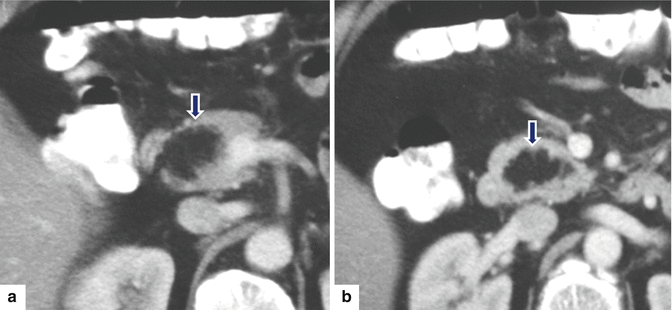
Fig. 13.19
Pancreatic lipoma. A 55-year-old female patient with an incidental pancreatic mass found on an abdominal CT performed to evaluate renal stones. CECT axial (a, b) images show a well-defined mass with fatty attenuation (–74 H.U.) in the pancreatic head (arrows)
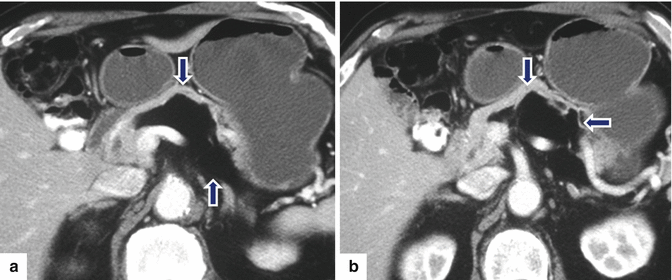
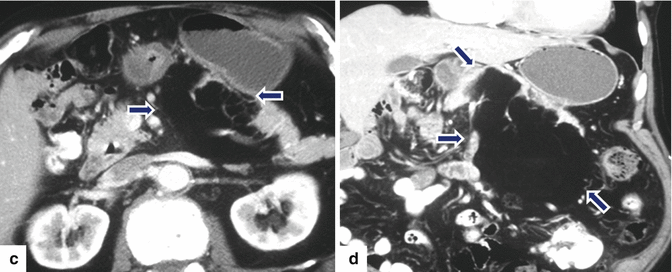
Fig. 13.20
Giant pancreatic lipoma. A 67-year-old asymptomatic male with history of a pancreatic lipoma that has been followed for several years and has shown stability. CECT axial (a–c) and coronal (d) images show a poorly marginated mass with fatty attenuation (–96 H.U) (arrows) involving the body and tail of the pancreas and extending into the root of the mesentery
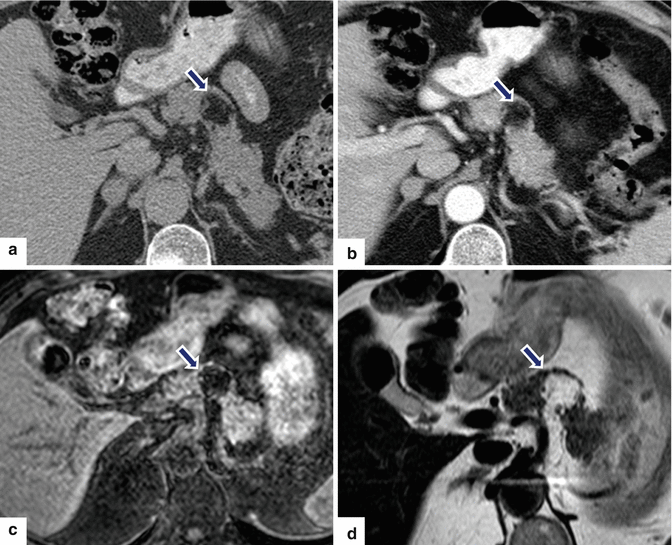
Fig. 13.21
Pancreatic lipoma. A 67-year-old male with history of right lower quadrant pain. CECT axial (a, b) images reveal a well-marginated mass of fatty attenuation in the pancreatic body (arrows). Fat-suppressed gradient echo T1-weighted (c) and T2-weighted axial (d) images confirm the presence of a fatty mass in the pancreas (arrows)
US: variable echogenicity. These masses can either be hyperechoic, isoechoic, hypoechoic or can have a mixed echogenicity.
CT: homogeneous, well-circumscribed, fat density mass (–80 to –120 HU without visible contrast enhancement).
MR: T1WI/T2WI: well-circumscribed mass with high signal intensity. Saturates in fat-suppressed sequences. Usually no signal decrease in out-of-phase sequences relative to in-phase with chemical shift imaging.
13.9.6 Differential Diagnosis
Focal fatty infiltration of the pancreas, pancreatic teratoma, and invasive liposarcoma
13.9.7 Treatment
Asymptomatic or incidental lipomas can be managed conservatively and monitored with serial imaging
Surgery recommended in symptomatic lipomas compressing on vital structures or lipomas with changes suggestive of malignancy (large size, rapid growth, or radiologically heterogeneous)
Practical Pearl
The majority of lipomas have characteristic features on imaging which allow for their differentiation from other lesions. Identification of such features, together with the lack of clinical symptoms, allows in most cases correct diagnosis without the necessity of histopathologic confirmation.
13.10 Granular Cell Tumor of the Pancreas (GCT) (Fig. 13.22)
Very rare, benign pancreatic neoplasm of Schwann cell origin.
Can be found in virtually every location of the body (breast, pituitary, central nervous system, respiratory tract, and gastrointestinal tract).
The characteristic epidemiology, clinical symptoms, and radiologic findings are difficult to describe due to rarity.
This tumor is extremely rare in the pancreas.
13.10.1 Macroscopic Appearance
Unencapsulated, ill-defined, yellow, firm mass
13.10.2 Microscopic Appearance
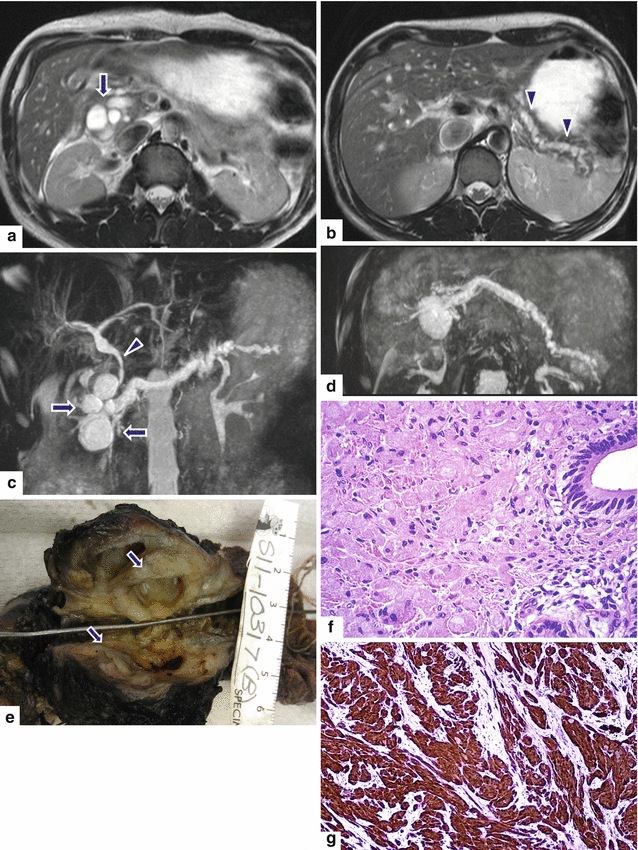
Fig. 13.22




Granular cell tumor surrounding the pancreatic duct. A 35-year-old male with history of chronic pancreatitis, jaundice, and chronic abdominal pain. Single-shot fast spin echo T2-weighted axial (a, b) images demonstrate multiple pseudocysts in the pancreatic head and diffuse irregular, dilatation of the pancreatic duct. MRCP coronal (c) and axial (d) images confirm the presence of multiple pseudocysts in the pancreatic head (arrows), dilatation of the pancreatic duct, and dilatation of the biliary system due to a long stricture involving the common bile duct (arrowhead). The patient underwent a Whipple procedure. Photograph of the fixed bivalved surgical specimen (e) demonstrates multiple cysts in the pancreatic head containing clear fluid (arrows). Histological section (f) shows a periductal mass, composed of large polygonal cells with abundant granular cytoplasm and small nuclei (H&E, 60×). The tumor cells are positive for S100 protein immunostain (g) (immunostain, 20×). Findings are consistent with granular cell tumor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree