Breast cancer is the most common cancer in women. One in 8 women develops breast cancer and approximately 30% of all affected women die of the disease. By performing a nationwide screening program in the Netherlands, a mortality reduction of 1.2% annually was achieved. The screening program is for women between the ages of 50 and 75 years; however, women with an increased risk for developing breast cancer are mostly younger. The role of MRI in this particular group of women has been described in different studies. MRI of the breast in this group of women has a higher sensitivity than mammography, but the highest sensitivity is reached by the combination of these two imaging modalities.
Breast cancer is a major health problem in Europe and the United States. At the moment, 1 in 8 women in the Western European countries develops breast cancer during her lifetime, and approximately 30% of these women die of the disease. Although a nationwide screening program for breast cancer has shown a mortality reduction of approximately 1.2% annually in the Netherlands, mammography has a limited sensitivity especially in the dense breast, and cancers are missed at screening.
In women with an increased risk for breast cancer, mammographic results are even more disappointing because in many cases these women are young, and younger women have often more dense breasts than postmenopausal women.
The first results regarding breast cancer screening with MRI were published by Tilanus-Linthorst and colleagues in 2000. In this series, MRI detected, in 3% of the cases, a malignancy not seen on conventional imaging. After that publication, many studies were published showing the value of screening with MRI and mammography compared with screening with mammography alone in the group of women with an increased risk for breast cancer.
Genetic counseling
There are many factors that influence a woman’s risk of developing breast cancer. Family history and increasing age are among the most significant risk factors. Approximately 20% to 30% of all women who have breast cancer have a relative with breast cancer. Five percent to 10% of all breast cancer cases are truly hereditary.
In performing risk assessment, family history is important as is the ethnic background of a patient. For instance, women of Ashkenazi Jewish origin have an increased risk of being BRCA1 or BRCA2 carriers compared with other ethnic groups. It is recommended that women with a likelihood of at least 10% of being a gene mutation carrier be referred for genetic counseling and testing ( Table 1 ).
| 1. One first-degree member |
| Breast cancer (BC) < 35 Bilateral or multicentric tumor, first tumor < 50 Ovarian cancer and BC < 70 Ovarian cancer < 50 Male BC Breast cancer |
| 2. Two or more relatives in the same side |
| Two first-degree with BC < 50 BC < 50 and ovarian cancer at any age Prostate cancer < 60 and first-degree BC < 50 Two relatives with ovarian cancer Three or more first-degree relatives, one < 50 |
There are different models used for risk assessment. The Cancer and Steroid Hormone Study is a population-based case-control study, often referred as the Claus model, that fits genetic models to derive age-specific breast cancer risk assessment for women with a first-degree relative with breast cancer. The assessment of risk is based on the relative’s age at diagnosis and the degree of relationship of these relatives.
Another often-used model is Gail and colleagues’ model. This model uses the following risk factors: age at menarche, age at first live birth, number of previous biopsies, presence of atypical hyperplasia, and number of first-degree relatives with breast cancer. There are some limitations to this model, for instance, the number of biopsies and the exclusion of more extended information about the family history. Evaluating a woman with both risk models in many cases gives discordant results.
BRCAPRO is a pedigree-based risk assessment model. BRCAPRO, however, only considers first- and second-degree family members.
Indications for performing MRI screening
Different guidelines have been published in the past decade providing indications for breast MRI in women with an increased risk for the development of breast cancer.
In the 1990s the first nonrandomized studies were initiated in the Netherlands, the United Kingdom, Canada, the United States, Italy, and Germany to determine the additional value of breast MRI to mammography in women who are BRCA1 or -2 carriers or have a lifetime risk of at least 20% to 25% for developing breast cancer. On the basis of these various retrospective studies, the American Cancer Society recommends annual MRI examination for all women with a lifetime risk of more than 20% to 25%. These guidelines are completely incorporated in the guidelines of the European Society of Breast Imaging, which describe in detail which groups of women should undergo breast MRI ( Box 1 ).
- 1.
BRCA mutation
- 2.
First-degree untested relative of BRCA carrier
- 3.
Radiation to chest wall between ages 10 and 30
- 4.
Li-Fraumeni syndrome and first-degree relatives
- 5.
Cowden syndrome and first-degree untested relatives
Indications for performing MRI screening
Different guidelines have been published in the past decade providing indications for breast MRI in women with an increased risk for the development of breast cancer.
In the 1990s the first nonrandomized studies were initiated in the Netherlands, the United Kingdom, Canada, the United States, Italy, and Germany to determine the additional value of breast MRI to mammography in women who are BRCA1 or -2 carriers or have a lifetime risk of at least 20% to 25% for developing breast cancer. On the basis of these various retrospective studies, the American Cancer Society recommends annual MRI examination for all women with a lifetime risk of more than 20% to 25%. These guidelines are completely incorporated in the guidelines of the European Society of Breast Imaging, which describe in detail which groups of women should undergo breast MRI ( Box 1 ).
- 1.
BRCA mutation
- 2.
First-degree untested relative of BRCA carrier
- 3.
Radiation to chest wall between ages 10 and 30
- 4.
Li-Fraumeni syndrome and first-degree relatives
- 5.
Cowden syndrome and first-degree untested relatives
BRCA1 and -2 mutation carriers
A BRCA1 mutation carrier has a lifetime risk, according the Breast Cancer Linkage Consortium (BCLC), of 87% for getting breast cancer. Brose and colleagues calculated a lower lifetime risk of approximately 73%. In the Ashkenazi Jewish women who are BRCA1 carriers, the lifetime risk is slightly lower, between 40% and 60%.
In the group of BRCA1 mutation carriers, other cancers are also more frequently detected. BRCA1 carriers have a 40% to 65% cumulative risk of getting a contralateral breast cancer and a 20% to 45% risk of developing ovarian cancer. Prostate cancer is probably also one of the malignancies associated with BRCA1, although less frequent than in BRCA2. Male breast cancer is also seen in BRCA1 families as well as pancreatic cancer. The lifetime risk for developing breast cancer in BRCA2 mutation carriers is slightly lower than in BRCA1 groups. The age at onset is slightly later. According to the BCLC, the lifetime risk in BRCA2 is 84%. A large meta-analysis pooled pedigree data, however, that included more than 8000 women revealed an average cumulative breast cancer risk to age 70 in BRCA1 carriers of 65% versus 45% in BRCA2 carrier groups. BRCA2 mutation carriers also have an increased risk for getting other cancers (similar to those described previously).
BRCA1-associated breast cancers are more frequently high grade and receptor status negative. Moreover, lymphocytic infiltration is seen more often. The tumors, even when small in size, are often growing with pushing borders. In contradistinction, BRCA2 breast cancer behaves more like the group of sporadic breast cancers. BRCA1 tumors often have medullary or atypical medullary features. Although ductal carcinoma in situ (DCIS) in and around the invasive ductal cancer is also detected in BRCA1 mutation carriers, it is less frequent than in sporadic invasive ductal cancers. Unlike sporadic breast cancers where the size of the tumor is a predictor for lymph node invasion, this is not the case in BRCA1 mutation carriers. Although the tumor characteristics of the BRCA1 carriers show unfavorable signs, currently there is no difference in survival between this group of women and women with a sporadic breast cancer.
Sporadic hereditary breast cancer syndromes
There are other, more sporadic hereditary diseases that have an increased risk for breast cancer. The Li-Fraumeni syndrome is an autosomal dominant disease causing an increased risk for developing different kinds of cancers, such as sarcoma, leukemia, and breast carcinoma. Approximately 30% of all malignancies in this syndrome develop before the age of 15, and by the age of 70 approximately 90% of the malignancies have occurred. The Li-Fraumeni syndrome germline TP53 mutation has an unusual high prevalence in parts of Brazil. The incidence there is 1: 300 individuals. Early breast cancer before age of 40 is often seen in this group of patients.
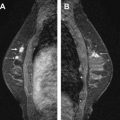
Cowden disease (multiple hamartoma syndrome) is also a rare genetic disease with an increased risk for developing breast cancer and for thyroid cancer ( Fig. 1 ).
Peutz-Jeghers syndrome is characterized by hamartomatous polyps in the small bowel and also malignancies of the gastrointestinal tract and breast. In Peutz-Jeghers syndrome, the lifetime risk for developing colon cancer is approximately 40%, for pancreatic cancer 35%, and for stomach cancer 30%. The risk for developing breast cancer is approximately 55%.
Muir-Torre syndrome is a variant of the hereditary nonpolyposis colon cancer. Women affected with this syndrome also have an increased risk for developing breast and endometrial cancer.
Ataxia telangiectasia is an autosomal recessive condition. Patients with this syndrome have immunodeficiency, cerebellar degeneration, and an increased risk for developing solid tumors, leukemia, and malignant lymphoma. They also have an increased risk for developing breast cancer.
Patients with a family history of breast cancer tend to develop breast carcinoma at a younger age than women with no family history. The prevalence of bilateral breast cancer is also higher than seen in sporadic cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree