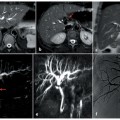
Degenerative joint disease (DJD) of the lumbar spine represents a complex pathologic process influenced by mechanical stress, structural degeneration, and inflammatory changes rather than normal aging. Imaging features of lumbar DJD are outlined, with emphasis on the importance of recognizing pathologic changes that contribute to patient symptoms. Additionally, recent advancements in the understanding and imaging of DJD are discussed.
Key points
- •
Degenerative joint disease of the lumbar spine should be considered a pathologic process, not a component of normal aging.
- •
Although patients’ symptomatology may not always correlate with imaging findings, known causes of acute and/or chronic back pain should be reported.
- •
Research-focused advanced imaging, including T1rho and T2 mapping, demonstrate potential to improve early detection of degenerative changes.
- •
Comprehensive radiologic reporting is essential for personalized, multidisciplinary care.
Introduction
Degenerative joint disease (DJD) of the lumbar spine is a progressive condition characterized by biochemical, structural, and biomechanical alterations in the spinal components, including intervertebral discs, adjacent vertebral elements, and facet joints. Structural alterations associated with DJD in affected components often appear similar across patients of varying age groups, and distinct from other preserved spinal segments within same individual, suggesting DJD of the lumbar spine to be a pathologic process rather than normal aging , ( Fig. 1 ). Despite its common occurrence in asymptomatic patients, DJD is seen more often in patients with low back pain and has been recognized as an important contributor toward significant pain and disability related to low back pain (LBP). , Imaging has always played an important role in the detection of DJD, assessing its role in patients’ symptoms, and assisting in management of patients after diagnosis. With the emergence of minimally invasive surgery and potential of newer targeted regenerative treatment including intradiscal injections of hydrogels and/or biological agents, this role is likely to grow further. ,

Anatomy of Lumbar Spine
In the absence of transitional anatomy, the lumbar spine consists of 5 vertebrae (L1–L5), intervertebral discs, facet joints, ligaments, and neural elements. These structures contribute toward 6 functional spinal units between T12-S1 segments designed to provide support, mobility, and protection for neural structures.
Each vertebra can be divided into the vertebral body, vertebral arch, and pars interarticularis. Vertebral bodies are cylindrical structures that bear weight and serve as attachment points for intervertebral discs. The vertebral arch forms the posterior boundary of the spinal canal, and consists of pedicles, laminae, and spinous process. The pars interarticularis is a key structural region between the superior and inferior articular processes, often affected by stress-induced injuries.
Intervertebral discs are composed of the nucleus pulposus, annulus fibrosus, and endplates. The central nucleus pulposus is a gelatinous core composed of proteoglycans and water, providing shock absorption. Surrounding the nucleus pulposus is the fibrocartilaginous annulus fibrosus, which offers structural containment and resists tensile forces. The endplates are composed of hyaline cartilage layers which connect the disc to vertebral bodies and facilitate nutrient exchange.
Facet joints are paired synovial joints between the superior and inferior articular processes of adjacent vertebrae, allowing for controlled movement and stability. Ligaments include the anterior longitudinal ligament, posterior longitudinal ligament, ligamentum flavum, interspinous ligament, and supraspinous ligament. These structures provide stability and limit excessive motion. The neural elements of the lumbar spine involve the spinal cord itself, which typically ends at L1-L2 (conus medullaris). Cauda equina nerve roots extend below the conus, with discrete nerve roots exiting through the neural foramina at each level.
Imaging Techniques
Accurate imaging is essential for identifying pathologic changes, delineating potential pain generators (eg, disc herniation, Modic changes), assessing stenosis severity, helping with patient management, and if possible—identifying specific cause for patients’ symptoms.
Despite its limited role in identifying many structural abnormalities associated with DJD, plain radiography is still used to screen for fractures and malalignment, identify variations in vertebral segmentation (thereby preventing interventions at a wrong level), and provide clinically useful information about functional stability across spinal segments during flexion and extension. Computed tomography (CT) allows high-resolution delineation of bony structures. While it is still limited in providing sufficient soft tissue details about degenerative changes in individual components of lumbar spine and their effect on underlying neural elements, it is useful to provide a roadmap for surgical planning prior to instrumented spinal fusions. In addition, along with discography, it has been used to provide morphologic information about the internal intervertebral disc architecture, while attempting to confirm/exclude the role of such changes in pain generation ( Fig. 2 ). However, its use has decreased more recently due to concerns about accelerated disc degeneration following discography. Magnetic resonance (MR) imaging provides a much superior soft tissue contrast allowing detailed visualization of intervertebral discs, nerve roots, spinal cord, and ligaments. With the additional benefit of not requiring exposure to ionizing radiation, MR imaging has become the key imaging modality to detect various pathologic changes associated with lumbar DJD. Clinically useful information can often be gathered using MR imaging without the use of intravenous contrast. Inclusion of fat-suppressed T2-weighted images such as short-tau inversion recovery (STIR) is, however, especially valuable in identifying bony changes (such as stress reaction and Modic type-1 endplate degeneration) as well as soft tissue changes (such as synovial cysts or periarticular inflammation around facet joints).

In addition to these routinely used clinical imaging methods, advancements in imaging have facilitated research to help improve our understanding of DJD and its clinical implications. Diffusion tensor imaging has the potential to allow further exploration of neural integrity. Functional MR imaging has been explored to study nociceptive and/or cognitive effects of chronic LBP produced by lumbar DJD on the brain. Quantitative techniques such as T1rho and T2 mapping can provide quantitative information about individual components of intervertebral discs such as endplate cartilage or the nucleus pulposus for a more objective detection of early disc degeneration. In addition, the use of positron emission tomography (PET) CT using agents such as 18 F-fluorodeoxyglucose and 18 F-sodium fluoride is being explored to identify specific nociceptive source in patients with low back pain.
Pathophysiology
Despite its wide prevalence, the pathogenesis of DJD is not fully understood. Interdependence of individual components of a functional spinal unit, however, means that the onset of pathology in one part of the unit often predisposes other components to undergo degeneration as well. Disc degeneration, a key component of DJD of lumbar spine, stems from imbalances in synthesis and degradation of extracellular matrix components. Longitudinal studies underscore a significant genetic predisposition to disc degeneration, with important contribution from environmental factors including mechanical stresses. , Various mechanisms including hereditary factors, reduced nutrient supply to the nucleus pulposus, and mechanical trauma have been proposed to be important in initiating disc degeneration. In vivo MR imaging-based cross-sectional studies in young adults and children have suggested that annular fissures and/or endplate defects, presumably caused by mechanical injury, often precede the appearance of nuclear degeneration. The importance of mechanical factors is further highlighted by experimental studies documenting progressive disc degeneration following either intentionally induced injury to the annulus fibrosus or exposure of the intervertebral disc to increased mechanical loading. Similarly, in humans, intervertebral discs in segments with stress-related bony injuries have been shown to have higher prevalence of disc degeneration. The nucleus pulposus of degenerated intervertebral discs demonstrate altered proteoglycan content, collagen fragmentation, and inflammatory mediator release, culminating in loss of structural integrity and altered biomechanics. , Recent advances in understanding DJD pathophysiology emphasize the role of proinflammatory cytokines like tumor necrosis factor (TNF)-α and interleukin (IL)-6 in perpetuating disc degeneration. These factors lead to matrix breakdown and drive endplate degeneration including Modic changes. , The presence of inflammatory response along the endplates is a particular hallmark of Modic type-1 change. Some researchers have invoked the possibility of indolent infectious etiology for some of these cases, a somewhat controversial hypothesis that is a focus of many ongoing research studies. ,
Degeneration of specific components of spinal segments
Intervertebral Discs
On T2-weighted images, hyperintense signal of a normal nucleus pulposus is easily recognizable in the central aspect of intervertebral discs. Loss of this hyperintensity (disc desiccation) is a relatively consistent hallmark of disc degeneration reflecting structural changes occurring within the nucleus pulposus. Severity of this signal can be assessed on grading systems such as one proposed by Pfirrmann and colleagues ( Fig. 3 ). While T2 mapping provides a much more objective quantitative assessment of such changes, it is still used mainly as a research tool. Annular fissures are visualized as foci of increased signal intensity on T2-weighted MR images within normally hypointense outer annulus fibrosus ( Fig. 4 ). The potential of partial volume averaging with adjacent tissues can make identification of small annular fissures difficult. While contrast-enhanced studies are not routinely performed in patients with low back pain, annular fissures often enhance following contrast administration (see Fig. 4 ), a finding that likely correlates to granulation tissue containing pain nerve endings seen on histologic sections across annular fissures.


Disc degeneration is often accompanied by alteration in the contour of the affected intervertebral discs with disc margin extending beyond the adjacent vertebral body. , A standardized terminology for such contour abnormalities has been proposed and should be used. The terms disc bulge and herniation are used to describe such contour abnormalities affecting greater than 25% and less than 25% of the entire intervertebral disc circumference, respectively ( Fig. 5 ). Disc herniation can be further characterized as disc protrusions (with the base of disc herniation wider than its height) or disc extrusion (with the height of disc herniation wider than the base). Disc extrusions can migrate within the epidural space. Disc herniations that have lost their connection with the parent disc are termed disc sequestration ( Fig. 6 ). Description of disc herniations in radiology reports should include their location along the disc circumference (central, subarticular, foraminal, or extraforaminal) and the indication of migration/sequestration so that appropriate steps can be taken during any surgical intervention. While the disc herniations themselves do not enhance, a rim of enhancement can be seen around them because of secondary inflammation and/or formation of granulation tissue (see Fig. 6 ). Rare instances of intradural migration of disc material have been reported in the literature.