Primary Tumor (T)
Tx: Primary tumor cannot be assessed
T0: No evidence of primary tumor
Ta: Noninvasive papillary carcinoma
Tis: Carcinoma in situ
T1: Tumor invades subepithelial connective tissue
T2 : Tumor invades muscularis
T3 (renal pelvis only): Tumor invades beyond muscularis into peripelvic fat or the renal parenchyma
T3 (ureter only): Tumor invades beyond the muscularis into periureteric fat
T4: Tumor invades adjacent organs or through the kidney into perinephric fat
Regional Lymph Nodes (N) a
Nx: Regional lymph nodes cannot be assessed
N0: No Regional lymph node metastasis
N1: Metastasis in a single lymph node, 2 cm or less in greatest dimension
N2: Metastasis in a single lymph node, more than 2 cm but not more than 5 cm in greatest dimension; or multiple lymph nodes, none more than 5 cm in greatest dimension
N3: Metastasis in a lymph node more than 5 cm in greatest dimension
Distant Metastasis (M)
Mx: Distant metastasis cannot be assessed
M0: No distant metastasis
M1: Distant metastasis
Stage grouping | |||
|---|---|---|---|
Stage 0a | Ta | N0 | M0 |
Stage 0is | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage III | T3 | N0 | M0 |
Stage IV | T4 | N0 | M0 |
Any T | N1 | M0 | |
Any T | N2 | M0 | |
Any T | N3 | M0 | |
Any T | Any N | M1 |
Many studies have demonstrated strong correlation between survival and tumor stage, grade, and multifocality, with staging being the most important factor [13]. The 5-year survival rate for patients with Ta or T1 disease ranges from 60 to 90 %, decreasing to only 5 % in T3 or T4 disease [14]. Furthermore, the histological grade (see Table 2.3) has also demonstrated close correlation with staging (e.g., superficial tumors tend to be of lower grade whereas higher grade tumors have higher propensity to be invasive) [15].
GX | Grade cannot be assessed |
G1 | Well differentiated |
G2 | Moderately differentiated |
G3-4 | Poorly differentiated or undifferentiated |
Table 2.4
Imaging techniques summary
Evaluation of patient with hematuria and staging |
Intravenous urography |
Largely replaced by CTU |
Examination of choice if CTU not available |
Does not provide information about surrounding structures |
CT urography |
Current standard for minimally invasive imaging |
Useful for staging and diagnosis |
Allows assessment in multiple planes |
Evaluates periureteric and renal infiltration |
Evaluates for nodal and distant metastasis |
Split bolus technique, with two runs may minimize radiation |
Prone positioning, intravenous saline bolus and oral water hydration help maximize ureteral opacification and collecting system distension |
Currently, there is no single universal standard CTU protocol. However, for the past 5 years there has been an increasing trend toward CTU techniques with improved ureteral opacification and minimal radiation dose. Radiation dose including cumulative dose from repeated examination is a specific point of consideration when utilizing CT urography in imaging work-up, particularly for younger patients who may undergo repeat follow-up examination. The effective dose for CTU is undeniably higher than that of conventional urography. Nawfel et al. [16] calculated an estimated effective dose of 14.8 mSv for CTU utilizing a single bolus three-phase technique compared to 9.7 mSv for conventional urography. However, Martingano et al. [17] demonstrated that CTU estimated effective dose may reach levels up to 17.1 mSv for nephrographic-excretory phase, but with optimization of radiation a value of 6.2 mSv was obtained. |
MR urography (MRU) |
Less spatial resolution compared to CTU |
Alternative for patients with iodinated contrast allergy |
Possible alternative in patients with mild renal insufficiency |
Exception: patients with severely impaired renal function, due to potential nephrogenic systemic fibrosis (NSF) |
Patients must be able to be exposed to the magnetic field without contraindications |
Pyelography |
Anterograde: Rarely performed when CTU is inconclusive for evaluation of renal pelvis because of theoretical risk of tumor seeding |
Retrograde: Indicated if poor renal function, contrast allergy or partial or incomplete visualization of the collecting system, however even then, MRU may prove a superior alternative when not contraindicated. |
Representative CTU protocol and technical factors |
IV injection: |
Saline 2.5 mL/s for 200 mL |
Contrast 2.5 mL/s for 150 mL (e.g., iohexol 300), followed by saline 2.5 mL/s 200 mL |
Oral contrast: |
400 mL H2O 20 min before scan |
Precontrast, parenchymal phase and excretion phase imaging: |
Injection to scan delay: parenchymal phase 80 s; excretion phase: 12 min |
Scan coverage: |
Precontrast: just above kidneys through ischial tuberosity |
Parenchymal phase: just above hepatic dome through tuberosity |
Excretion phase: same as precontrast |
Technical factors: |
Exposure: |
Precontrast and excretion phases: 0.7 s rotation time; low mA (e.g., 130) |
Parenchymal phase: 0.7 s rotation time; auto mA |
Pitch/table speed: all phases, 0.984/39.37 mm |
Reconstruction thickness: 2.5 × 2.5 mm standard all phases |
Reconstruction 2: 1.25 × 1 mm standard |
Additional delays: |
Only scan targeted 12 min supine and/or 18 min prone, if necessary |
Additional delays will be determined by the radiologist, if pathology is suspected |
(Abdominal compression: none) |
Reformats: |
Parenchymal phase: |
1.6 × 0.8 mm coronal reformats through kidneys, ureters, and bladder |
3D reconstruction: use 1.25 × 1 mm reconstructions from excretion phases to push to workstation |
Studies suggest that renal pelvis tumors tend to have better prognosis than ureteral tumors; this is thought to be due to the renal parenchyma acting as a barrier [2, 11].
Treatment and prognosis are largely dependent on the depth of tumor infiltration, the degree of lymph node involvement, metastases, and the histology of the tumor, further emphasizing the need for proper staging.
After the detection of hematuria or diagnosis of suspected upper tract TCC, radiological evaluation is important in detecting and determining tumor extent and stage (see Table 2.4). Intravenous urography has been largely replaced by computed tomography urography (CTU), capable of detecting lesions larger than 5 mm [18], as this technique can image the entire urinary tract and provide information on locoregional extension, as well as nodal and distant metastases. However, not all filling defects within the collecting system represent urothelial tumors. Although rare, other malignant lesions such as sarcomas (leiomyosarcoma, Ewing’s, liposarcoma, rhabdomyosarcoma) and benign conditions (e.g., blood clot, fungus ball, calculi, sloughed papilla, malakoplakia, tuberculosis, ureteritis cystic, and endometriosis) may present in a similar fashion, making differentiation more challenging, sometimes requiring endoscopy and biopsy for differentiation before treatment.
Magnetic resonance imaging (MRI) urography is not currently considered standard due to its inferior spatial resolution to CT, long scanning time and cost [19]. However, it remains an attractive alternative technique that does not involve ionizing radiation. Currently, MR urography is a noninvasive alternative that has found use predominantly in patients allergic to iodinated contrast media.
Management
Traditionally, organ-confined renal pelvis and proximal upper ureteral disease in patients with otherwise normal contralateral kidney has required radical nephroureterectomy with resection of bladder cuff [20]. There are several reasons for this: the synchronous and metachronous nature of TCC, the high tumor recurrence rate (30–50 %) [21] within the ureteral stump when only a nephrectomy is performed, and the low frequency of contralateral lesions (1–5.8 %) [22].
Regional lymphadenectomy is then performed, especially for patients with high-grade tumors; however, the role and extent of routine lymphadenectomy are still to be determined [23].
Segmental ureteral resection with anastomosis or uretero-neocystostomy for proximal or midureteral tumors and distal ureterectomy for tumors of the distal ureter without evidence of multifocality are feasible options, especially for low-grade, low-stage lesions, and in patients in whom renal function preservation is vital.
There is an increasing trend toward conservative management with recent advances in endoscopic technology and minimally invasive surgery. Such conservative management allowing for renal function preservation is generally accepted for patients with low-grade tumor, solitary kidney, bilateral disease, poor renal function, or comorbidities precluding open surgery [24].
Despite no accepted protocol for radiological follow-up, endoscopic and cytological surveillance for upper urinary tract TCC is essential, irrespective of the surgical procedure employed, to detect local recurrence.
Due to its rarity, recommendation on topical chemotherapy, immunotherapy, and neoadjuvant systemic chemotherapy of upper tract disease extrapolates from the management of lower urothelial tract tumors. Controversy still exists on the role of radiotherapy and chemotherapy in reducing tumor recurrence and lengthening survival, and further confirmation in randomized multicenter trials is needed to ensure efficacy [25].
Get Clinical Tree app for offline access

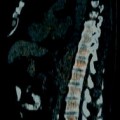
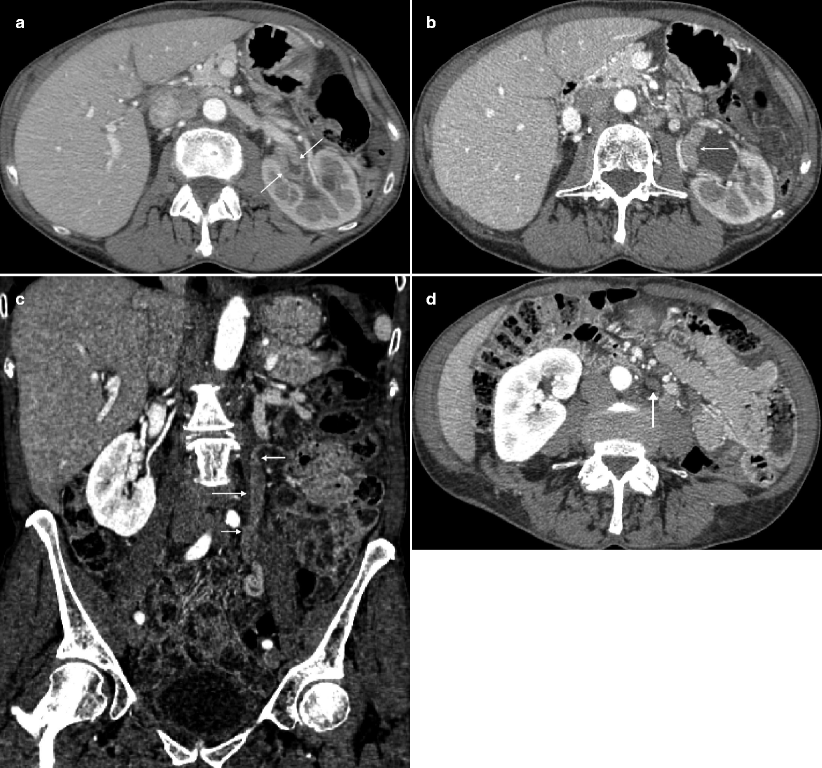
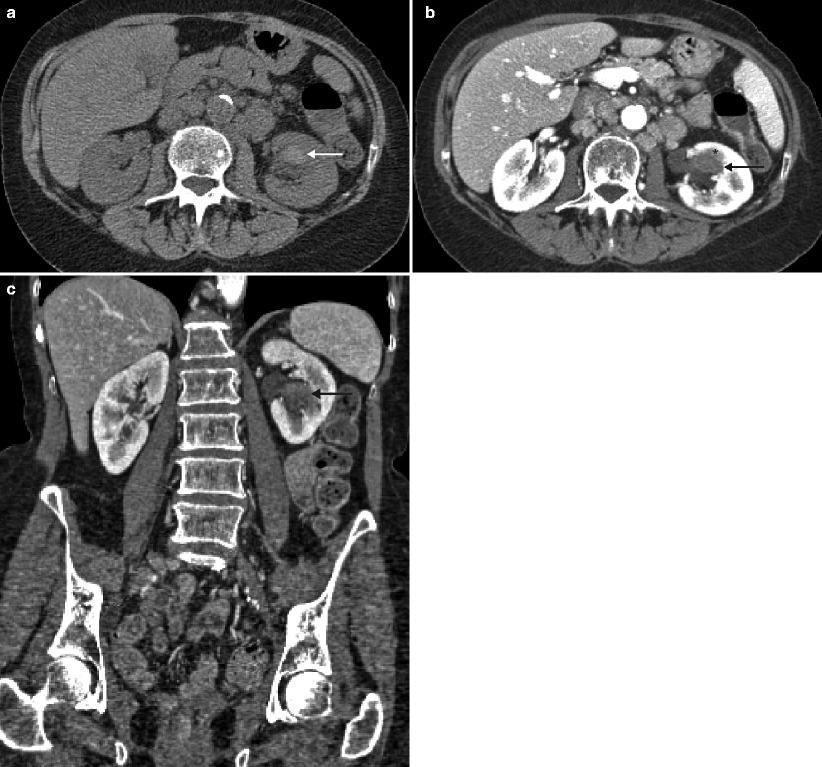
Fig. 2.1
A 68-year-old woman with a history of hematuria with bladder cancer after transurethral resection of bladder tumor (TURBT). CTU demonstrated extensive upper tract tumor and borderline enlarged retroperitoneal lymph nodes. T1 papillary involving the lamina without muscularis invasion was confirmed by histopathologic analysis. (a) Axial parenchymal phase images showed multifocal enhancing tumor within the left renal pelvis (arrows), as well as involving the upper moiety and upper ureter with moderate hydronephrosis. (b) Axial parenchymal phase images at a lower level show eccentric thickening (arrow) along the ureteropelvic junction. (c) Coronal multiplanar reformatted (MPR) showing the extent of urothelial tumor involving the left ureter (arrows) in this patient who also had a delayed nephrogram. (d) Axial parenchymal phase images at the level inferior to the left lower pole show prominent, borderline-enlarged para-aortic nodes (arrow). Node-positive tumors of the renal pelvis can involve upper retroperitoneal nodes (retrocrural, suprahilar, paracaval, paraaortic, and interaortocaval) and extend caudally to the external iliac lymph nodes. Node-positive tumors of the middle and lower third of the ureter can involve both the retroperitoneal and pelvic lymph nodes. Lymph node metastases have consistently been associated with an adverse prognosis [23]

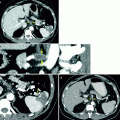
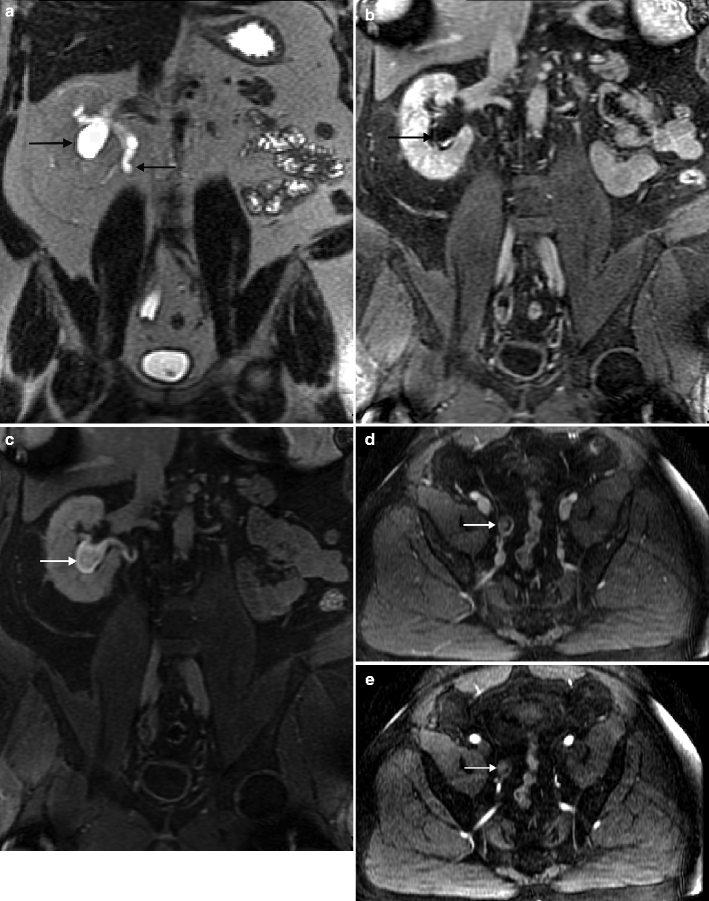
Fig. 2.2
A 55-year-old man with history of low-grade TCC involving the bladder and left upper tract after TURBT and left nephroureterectomy. Routine follow-up MRU revealed new right hydroureteronephrosis and lobulated enhancing lesion obliterating the distal ureteral lumen. Although ureteral washings at time of placement of JJ stent were negative for malignant cells, histopathology from subsequent right distal ureterectomy confirmed noninvasive urothelial carcinoma. (a) Coronal T2 image shows dilated renal collecting system and proximal hydroureter (arrows) or a variant of “champagne glass sign” related to more distal ureteral disease. Coronal, postintravenous, gadolinium-enhanced early (b) and excretory phase (c) imaging demonstrates a dilated lower pole moiety with confirmed nonenhancing filling defect consistent with debris (arrow). (d) Axial T1 fat-saturated postintravenous gadolinium images show distal ureteral enhancing tumor (arrow). (e) Distal ureteral tumor confirmed on subtraction images (arrow). Patients with a history of lower or upper tract urothelial carcinoma are at high risk of developing synchronous or metachronous urothelial carcinoma in the upper tract. MRU is a promising alternative for CTU in the evaluation of upper tract urothelial carcinoma, especially when the patient has a contraindication to iodinated contrast material. When the lesions are obstructive, MRU can detect upper tract urothelial carcinoma with an accuracy of 88 % or higher using gadolinium-enhanced pyelographic phase or T2-weighted single-shot fast spin-echo MRU but has a limited role in accurately staging low-volume tumor (Ta, T1, T2), which may be important in deciding the treatment option (nephroureterectomy versus endoscopic treatment) [26]


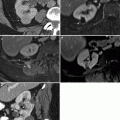
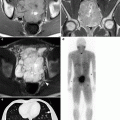
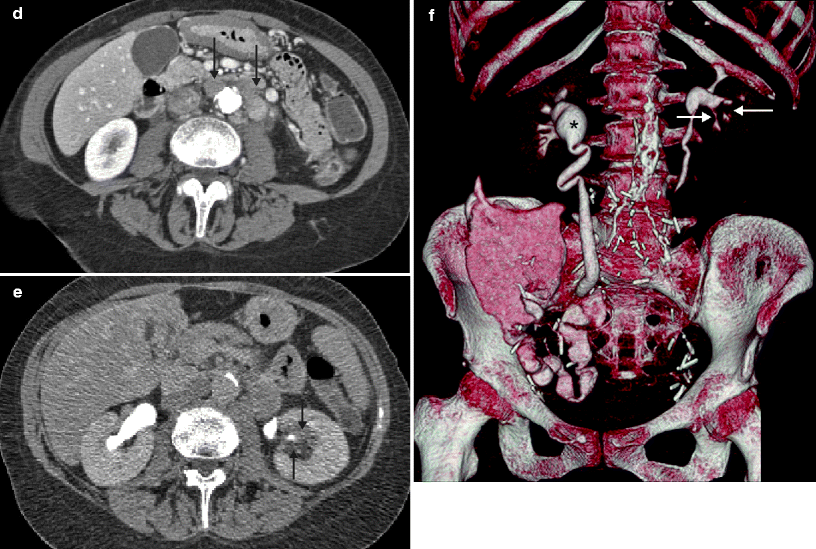
Fig. 2.3
A 69-year-old woman with history of pT3N2 bladder cancer after neoadjuvant chemotherapy and radical cystectomy found to have renal mass and adenopathy on routine follow-up CTU. The patient was managed with adjuvant chemotherapy. (a) Axial precontrast images show a hyperattenuating mass (arrow




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree