CHAPTER 19 Ureters, Bladder, and Urethra
ANATOMY OF THE URINARY TRACT
Anatomy of the Bladder
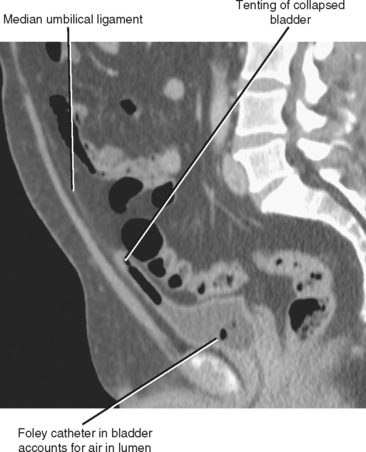
Early in development, the bladder empties through the umbilicus through a tube called the urachus. The urachus involutes in utero to form the median umbilical ligament, a long cord just superficial to the peritoneum that stretches from the bladder dome to the umbilicus. In the sagittal plane, the collapsed bladder dome is usually tented up anteriorly by its attachment to the median umbilical ligament (Fig. 19-1). A variety of abnormalities occur when obliteration of the lumen of the urachus is incomplete.
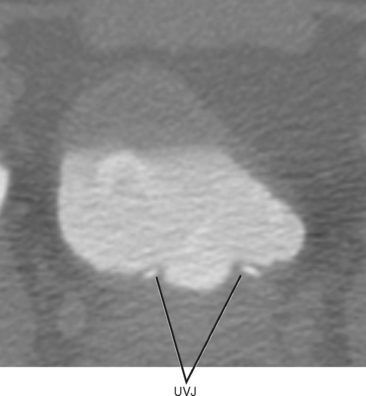
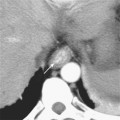
The interureteric ridge is a promontory on the posterior bladder wall between where the ureters enter the bladder. The uterovesical junction (UVJ) describes the short segment of ureter that courses through the muscular bladder wall, often protruding slightly into the bladder at the trigone (Fig. 19-2). These small normal protuberances of the UVJ are a helpful landmark when searching for ureteral jets by ultrasound.
Anatomy of the Male Urethra
Male urethral anatomy is slightly more complex than that of the female urethra, although familiarity with several landmarks facilitates accurate identification of normal anatomic structures. From the bladder to meatus, the male urethra is divided into four main segments: prostatic, membranous, bulbous, and penile (Fig. 19-3). Combined, the prostatic urethra and membranous urethra make up the posterior urethra; the bulbous and penile comprise the anterior urethra.
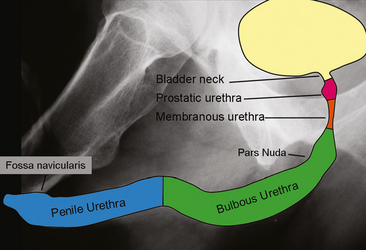
Figure 19-3 Illustration superimposed on a retrograde urethrogram demonstrates anatomic segments of the male urethra.
NORMAL IMAGING APPEARANCE OF THE URINARY TRACT
Normal Imaging Appearance of the Ureters
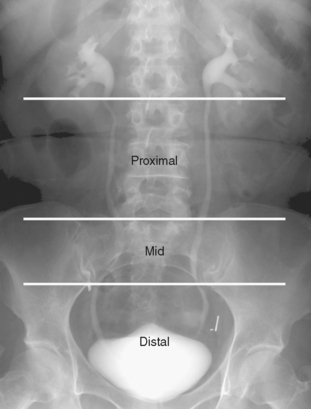
The ureters are usually divided into proximal, middle, and distal segments when describing abnormalities. Although radiologists often apply these terms loosely, a methodical system is commonly used by urologists when planning interventions. The proximal ureter refers to the segment from the UPJ to the superior margin of the sacrum, the midureter is the segment that overlies the sacrum (also referred to as the sacral ureter), and the distal ureter is the short segment between the inferior margin of the sacrum and the ureteral orifice (Fig. 19-4).
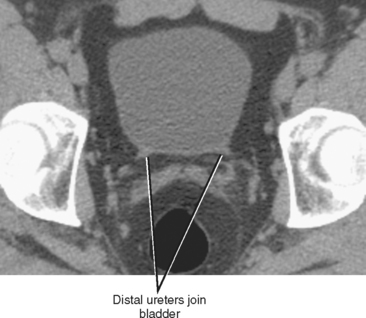
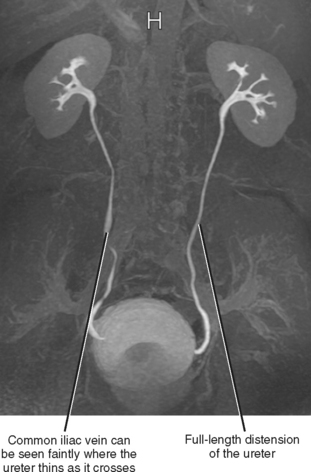
It is sometimes difficult to continue following the ureters as they cross anterior to the iliac vessels, particularly if there is a paucity of fat in the retroperitoneum. One remedy is to identify where the ureters enter the bladder at an angle of approximately 45 degrees (Fig. 19-5). The ureters can then be followed back (a retrograde noncontrast-enhanced computed tomography [NCCT] study!) to the level of the iliac vessels. With practice, one may become as comfortable following each ureter retrograde as antegrade, allowing a stone protocol CT examination to be viewed in a “U” configuration, following one ureter from kidney to bladder and the other ureter from bladder to kidney.
On contrast-enhanced CT, the normal ureter may enhance slightly in the arterial and portal venous phases. Excretion of contrast into the ureters usually starts by about 2 minutes after injection. The high attenuation of excreted contrast defines the ureteral lumen well but can obscure the thin ureteral wall. Coronal images (often using thin-slab maximum intensity projection [MIP]) provide visualization of longer segments of the ureters, and even small abnormalities can be detected on coronal reformations using isotropic data. Using a thicker MIP technique allows longer segments to be viewed on fewer coronal sections but also limits detection of small filling defects.
Administering a low dose of a diuretic such as furosemide (typically 5-10 mg) aids in ureteral distention and dilution of excreted contrast media. This reduces the negative effects that concentrated gadolinium has on signal intensity and permits contrast-enhanced excretory urography to be performed with MRI. Excretory-phase, gadolinium-enhanced, T1-weighted images can be acquired in the coronal plane to create an MR urogram (Fig. 19-6). Diuretic administration also benefits CT urography by distending the collecting systems and dispersing excreted contrast material more evenly.
Normal Imaging Appearance of the Bladder
MRI provides excellent characterization of the bladder, even without contrast media. The urine is bright on T2-weighted images, providing excellent contrast with the mucosal surface. Similar to difficulties with very-high-attenuation contrast on CT, it is often helpful to window the urine to a gray level to avoid obscuring subtle features of the bladder wall on T2-weighted images. T1-weighted images performed after gadolinium-based contrast injection but before the excretory phase allows detection of subtle enhancement in the normal bladder wall, as well as brighter enhancement in mucosal lesions. Once the excreted contrast material fills the bladder, a stratified appearance is common, particularly on delayed images. This appearance is not related to true contrast layering, but rather results when thresholds in concentration are reached that result in relatively abrupt changes in signal intensity despite gradual changes in gadolinium concentration.
Normal Imaging Appearance of the Female Urethra
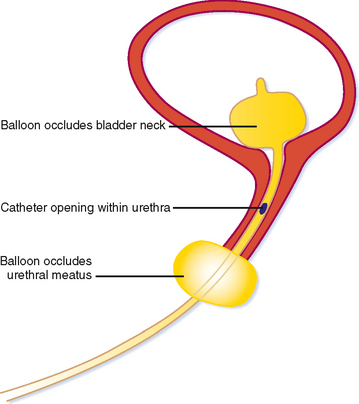
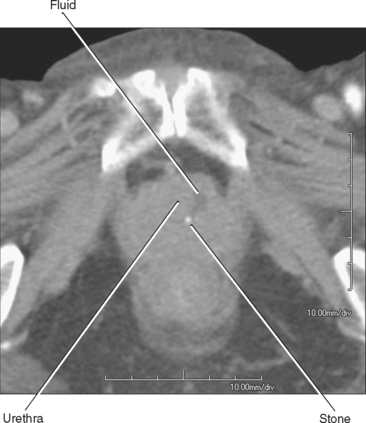
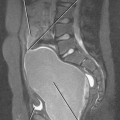
Because of its short length, retrograde urethrography (RUG) of the female urethra is not a simple matter. A balloon within the lumen would occupy much of its length and contrast is likely to fill the bladder preferentially, leaving the urethra poorly distended. When necessary, these challenges can be overcome with a double-balloon urethrogram. This requires a bladder catheter designed specifically for this purpose (Fig. 19-7). Fortunately, the urethra usually can be imaged effectively with cross-sectional imaging. With careful inspection, urethral diverticula can be seen with CT, particularly if they contain stones (Fig. 19-8). Large masses can also be seen with CT if they enlarge the urethra or invade adjacent structures.
The female urethra is best demonstrated with MRI, particularly when an endovaginal coil is used to produce high-resolution images. On axial sections obtained with an external surface coil, the urethra is posterior to the pubic symphysis and anterior to the vagina, contained within an inverted cone-shaped space created by the puborectalis muscle. The anus is situated at the apex of that inverted cone (Fig. 19-9).
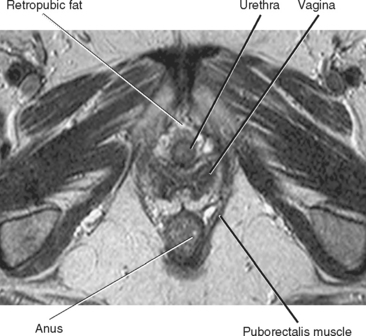
Figure 19-9 Axial T2-weighted magnetic resonance image of the female perineum demonstrates normal anatomic structures.
Normal Imaging Appearance of the Male Urethra
VCUG is the most commonly performed imaging technique for evaluation of the male urethra (Fig. 19-10). During voiding, the bladder neck opens, and the prostatic and membranous urethra widen. The bulbous and penile urethra usually become moderately distended and are typically of uniform caliber.
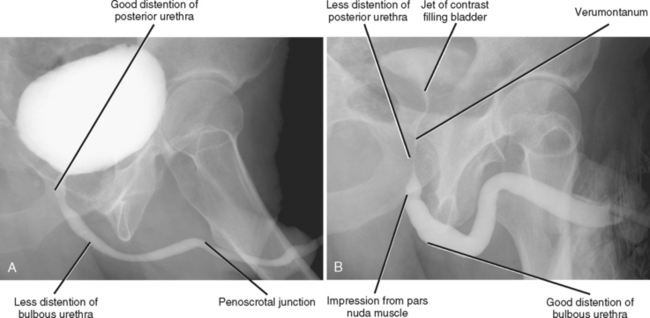
Figure 19-10 Normal male urethral anatomy demonstrated by (A) voiding cystourethrography and by (B) retrograde urethrography.
RUG is appropriate in the setting of trauma or suspected stricture. A Foley catheter is inserted into the urethra, and the balloon inflated with approximately 1 to 2 ml saline in the fossa navicularis. Alternatively, a catheter tip syringe can be inserted gently into the urethral meatus until snug. With the patient in an oblique position (providing relatively uniform body density throughout the length of the urethra by superimposing it over the thigh), water-soluble contrast is injected under fluoroscopy and images are obtained. The penile and bulbous segments are distended more with RUG than with VCUG (see Fig. 19-10). The posterior urethra, however, is generally less distended. A jet of contrast is often seen mixing with unopacified urine within the bladder and can have a bizarre appearance to the uninitiated.
CONGENITAL ABNORMALITIES
Duplication
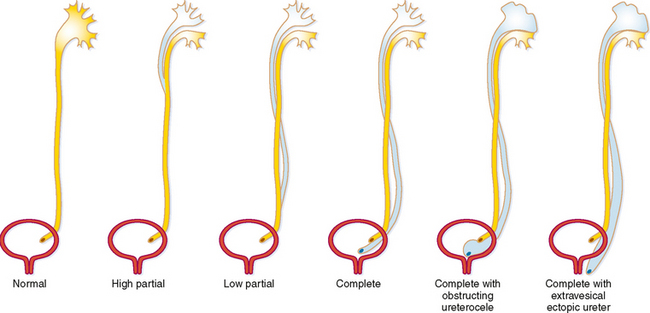
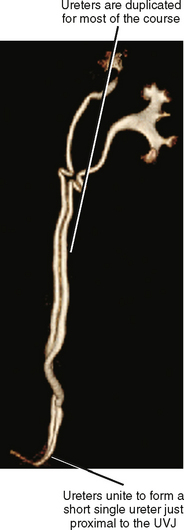
During fetal development, part of the mesonephric duct forms a ureteral bud that migrates cranially. When the ureteric bud reaches the metanephric blastema, it branches into multiple divisions and induces development of the kidney around a branched collecting system. Early branching or complete duplication of the ureteric bud results in duplication of all or part of the affected renal collecting system and ureter. If the bud divides completely at its origin, duplication is complete (two ureteral orifices). Any division of the ureteric bud proximal to the bladder trigone leads to variable lengths of duplication between the renal pelvis and the bladder (Fig. 19-11). Some form of duplication is present in up to 4% of people, and although it is usually an incidental radiographic finding, it occasionally has significant health implications.
Partial duplications that unite to form a common ureter rarely cause symptoms (Fig. 19-12). However, it is useful to have knowledge of duplicated anatomy in the event of procedures that place the ureters at potential risk, such as donor nephrectomy, pelvic surgery, or radiofrequency ablation. On occasion, partial duplication of the ureters (probably paired with abnormalities of peristalsis) results in channeling of urine down one ureter and up the other, so-called yo-yo reflux.
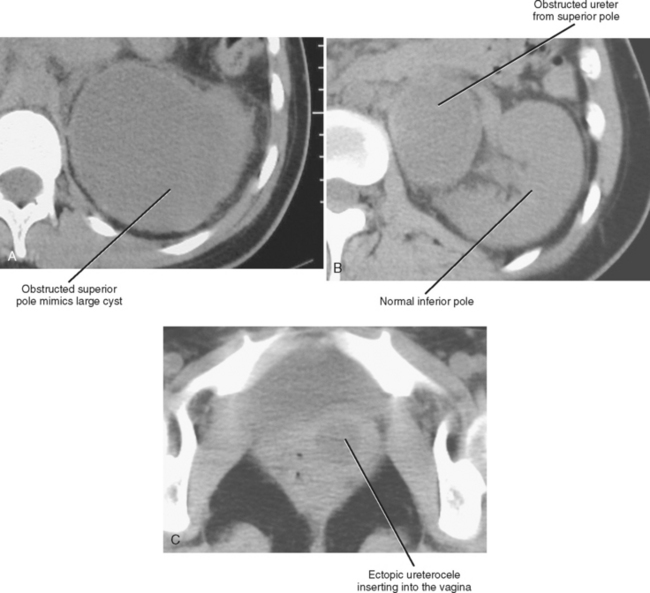
Most cases of medically significant duplication are complete. With complete duplication, the ureter from the lower pole usually inserts into a normal location in the bladder trigone. The ureter from the superior pole is ectopic, usually entering the bladder inferior and medial to the expected location (Weigert–Meyer rule). The most common location for the ectopic upper pole ureter is near or within the bladder neck. Even with complete duplication, if the ectopic ureteral orifice has normal morphology, it is not usually of clinical significance. However, when an ectopic ureter protrudes or herniates into the bladder lumen because of an abnormal angle of entry, it forms a ureterocele. Ectopic ureteroceles are prone to obstruction (Fig. 19-13). In addition, mass effect from a large ectopic ureterocele can distort the orifice of the orthotopic ureter, resulting in reflux to the lower pole.
The ectopic ureter from the upper pole can also insert into the urethra, vagina, prostate, or seminal vesicle. Female individuals with a ureteral orifice in the vagina or distal urethra suffer from incontinence. This leads to early diagnosis and favors preservation of some function in the upper pole moiety. Male individuals are more likely to have continent ectopia, with insertion into the prostate, seminal vesicle, or vas deferens. By the time many men are diagnosed with ectopic ureteral insertion, the upper pole moiety has severely compromised function because of obstructive uropathy.
Ureterocele
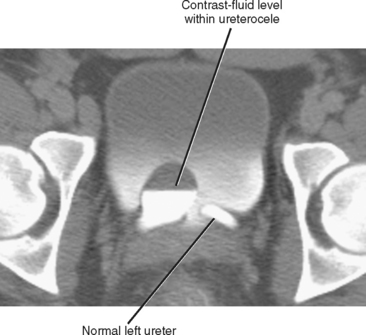
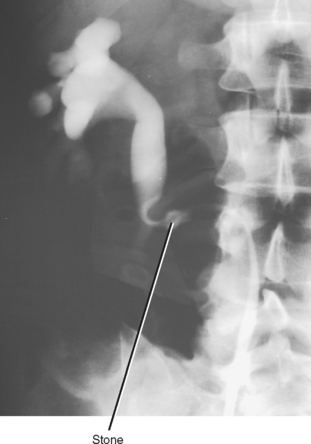
Simple ureteroceles occur without duplication and are located at the expected location of the normal ureteral orifice. Sometimes called orthotopic ureteroceles, these represent incomplete obliteration of the embryologic membrane between the ureter and bladder (Chwalla membrane). The flow of urine pushes the membrane, like wind filling a sail, causing the end of the ureter to push into the bladder. Simple ureteroceles are usually small and incidental (Fig. 19-14). Larger ureteroceles are prone to obstruction and stone formation (Fig. 19-15).
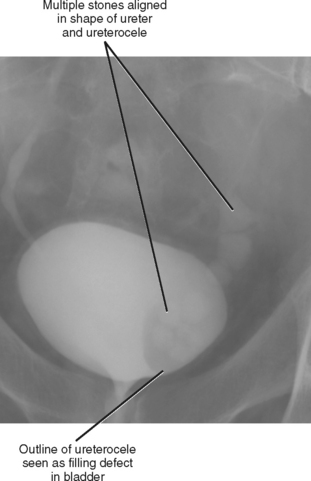
Figure 19-15 Images from an excretory urogram show multiple stones within a ureterocele and distal ureter.
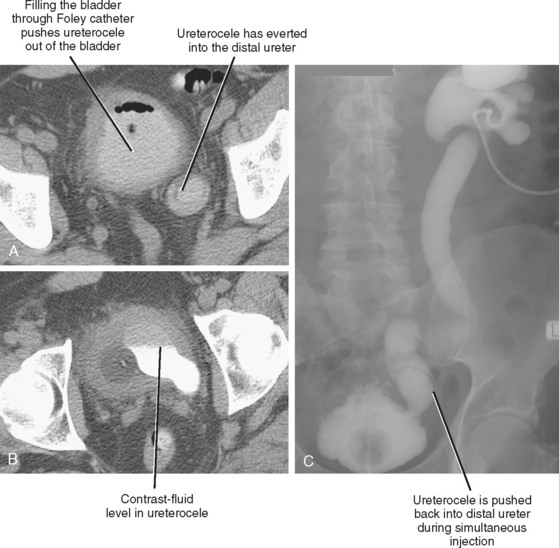
An everting ureterocele can occur when a ureterocele coexists with any cause of increased bladder pressure (usually benign prostatic hypertrophy). In such cases, the increased pressure within the bladder pushes the ureterocele back into the ureter, resulting in distal ureteral obstruction. On CT, an everting ureterocele results in the appearance of a membrane within the lumen of the ureter (Fig. 19-16). Because antegrade urography can push the ureterocele down into the bladder, combined antegrade urography and cystography may be required to confirm the diagnosis.
Ectopic Ureter
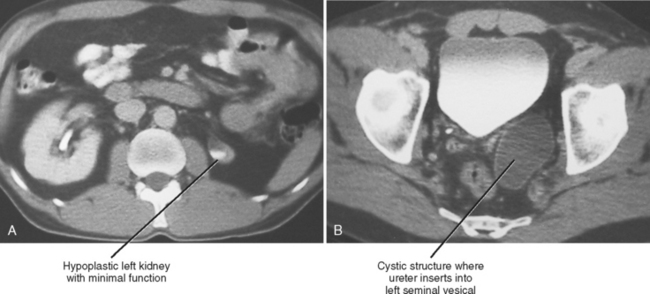
Just as ureteroceles can occur either with duplication or as an isolated abnormality, ectopic ureters can also occur without associated duplication. Isolated ureteral ectopia follows the same sex rules as duplication. Female individuals have a low insertion (bladder neck, urethra, or vagina) with associated incontinence and infections. In male individuals, ectopic ureters insert into the prostate, seminal vesicle, or vas deferens and can cause recurrent episodes of epididymoorchitis. In up to 5% of ectopic ureters, no ureteral orifice is found and the ureter is assumed to be a blind-ending pouch. Most ectopic ureters cause obstruction. When the system is not duplicated, obstructive nephropathy damages the entire kidney. Because onset of the insult is in utero, kidneys can be dysplastic or hypoplastic when discovered (Fig. 19-17).
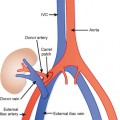
Circumcaval and Retrocaval Ureter
The portion of the inferior vena cava (IVC) below the renal vein usually develops from the supracardinal vein, which is posterior to the ureter. If, instead, the infrarenal IVC develops from the right subcardinal or postcardinal veins (these are anterior to the ureter), part of the ureter may become trapped behind the IVC. In the most common form of this variant, the proximal right ureter courses posterior to the IVC at the L4 level, and loops around medial then anterior to the IVC before assuming a normal location for the distal segment. This loop around the IVC is cause for the name circumcaval ureter. This may give the illusion that the ureter is “hung up” on the L4 pedicle (Fig. 19-18). This anatomic variant often presents when a ureteral stone becomes obstructed in the circumcaval segment (Fig. 19-19). This can be confusing on unenhanced CT if a calculus is lodged posterior to the IVC. Following the ureters carefully from kidney to bladder on sequential axial images allows accurate diagnosis.
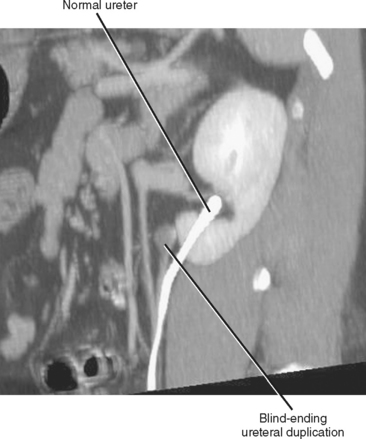
Blind-Ending Ureteral Duplication
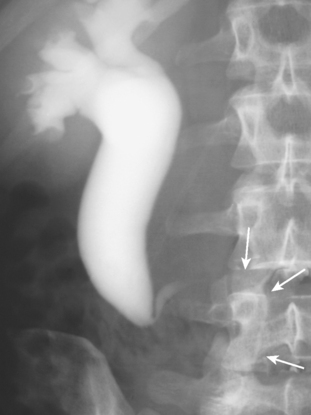
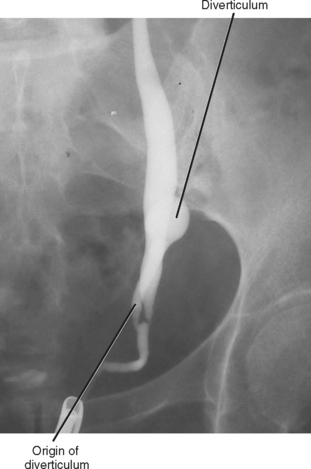
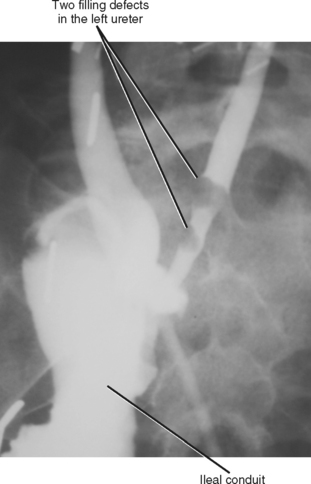
Refluxing excreted contrast media can opacify a blind ending ureteral duplication during excretory urography, but these anomalies are often better opacified on retrograde urography (Fig. 19-20). In some cases, expansion of the blind-ending ureter caused by reflux results in misdiagnosis as an ovarian cyst. Today, blind-ending ureters are more likely to be discovered with CT, although detection requires a methodical approach. Similar to identification of the ureter and gonadal vein on every examination, it is important to follow any dilated tubular structure in the retroperitoneum to confirm its beginning and end to avoid confusing a diverticulum with an obstructed ureter (Fig. 19-21).
Urachal Anomalies
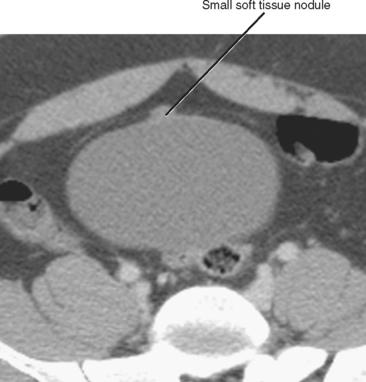
The urachus is a tube that empties the bladder through the umbilicus during early fetal development. The urachus usually narrows and then closes late in development or at birth. The closed urachus becomes the median umbilical ligament, an extraperitoneal cord that extends from the bladder dome to the umbilicus between the transversalis fascia and parietal peritoneum. The median umbilical ligament can be seen on virtually every multidetector computed tomography examination of the pelvis. Minimal soft-tissue thickening, trace calcification, or both are commonly present in the bladder dome where the median umbilical ligament inserts (Fig. 19-22).
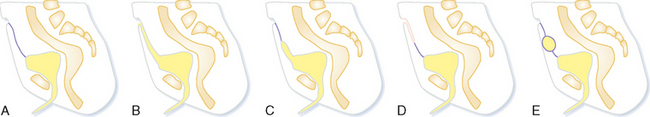
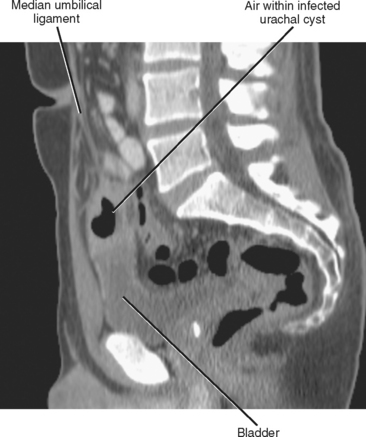
Failure of urachal closure may be complete (patent urachus), may involve the bladder end (urachal diverticulum), may involve the umbilical end (urachal sinus), or may be sequestered in the middle (urachal cyst) (Fig. 19-23). A patent urachus presents with urine leaking from the umbilicus. Urachal diverticula often occur when urachal closure is impeded by bladder outlet obstruction. Urachal sinuses and cysts usually present when they become infected (Fig. 19-24).
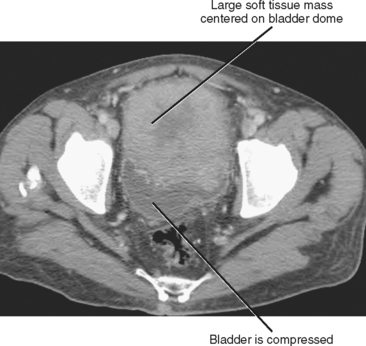
Urachal carcinoma is the most dreaded consequence of persistent urachal tissue. Tumor can arise anywhere along the urachal remnant but most commonly occurs just anterior to the bladder dome. Adenocarcinoma accounts for 80% of cases, with urothelial and squamous cell carcinomas occurring considerably less often. Hematuria is absent when the tumor does not communicate with the bladder lumen. Because no vital structures are compressed by a mass in this region, urachal carcinomas are usually large at presentation (mean size, 8 cm), and initial symptoms may be related to a palpable anterior pelvic mass or metastatic disease. Masses range from solid to low attenuation on computed tomography, often with heterogeneous areas of contrast enhancement (Fig. 19-25). Dystrophic calcifications are common.
FILLING DEFECTS AND IMPRESSIONS
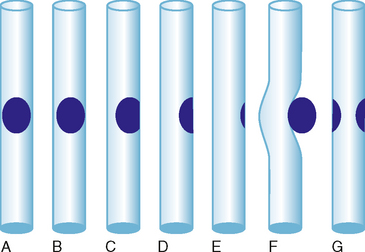
For the purposes of discussion, we define a filling defect as a space-occupying lesion that prevents contrast from completely filling the lumen of a hollow organ, thus altering its radiographic appearance (Fig. 19-26). We consider an impression to be a contour abnormality at the interface between intraluminal contrast and the wall of the lumen. Unlike filling defects that are surrounded by contrast, impressions bulge into fluid but are not engulfed by it. This appearance is often the result of superficial mucosal lesions, submucosal lesions, and extrinsic structures. This section includes filling defects and impressions together because the imaging appearance can overlap, and some entities (e.g., urothelial carcinoma) can have either appearance. Also keep in mind that some entities are neither filling defects nor impressions but can create a similar appearance (e.g., aberrant renal papilla).
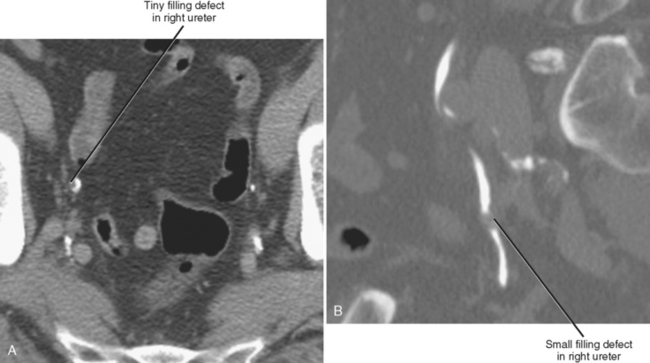
Filling defects within the urinary tract are most often the result of material passing through the urinary tract (e.g., stone, blood clot) or polypoid mucosal lesions (e.g., urothelial carcinoma, polyp) (Figs. 19-27 and 19-28). Impressions usually result from normal anatomic structures (e.g., blood vessels, bowel, uterus, prostate) and adjacent mass or, in the case of bladder impressions, extrinsic fluid collections. Table 19-1 summarizes some common filling defects and impressions that involve the urinary tract.
Table 19-1 Filling Defects and Impressions of the Ureters and Bladder
| Causative Factors | Distinguishing Features |
|---|---|
| Ureters and Bladder | |
| Stone | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|