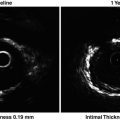
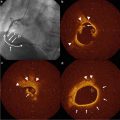
Fig. 4.1
The three-layered appearance of a cross sectional coronary artery as assessed by IVUS. I intima, M media, A adventitia, L lumen, C IVUS catheter
There are two different types of IVUS transducer: mechanical single element rotating transducer and the solid state electronic phased array transducer [17, 18, 21, 22]. The mechanical system utilises a single rotating transducer which is driven by a flexible drive cable at 1800 rotations per minute to sweep a beam almost perpendicular to the catheter. It offers a greater resolution owing to its higher frequency system and also a more uniform pullback. This system is available commercially as the 40 MHz iCross or Atlantis SR Pro catheters (Boston Scientific, Santa Clara, CA), the Revolution 45 MHz catheter (Volcano Corp, Rancho Cordova, CA), and the 40 MHz Lipiscan IVUS (InfraReDx, Burlington, MA). The electronic phased array system, on the other hand, uses multiple transducer elements which are arranged in an annular array and sequentially activated to generate an image. Commercially, it is available as 5 Fr 20 MHz Eagle Eye catheter (Volcano Corp). Benefits of this system include enhanced trackability and lack of non-uniform rotational distortion artefacts. The latter is unique to mechanical system and due to mechanical binding of the drive cable [23].
Clinical Application
Evaluation of Ambiguous Lesions in Non-Left Main Coronary Artery
Assessment and management of angiographic intermediate lesion (50–70 % stenosis) is one of the clinical dilemmas that is faced by an interventional cardiologist especially when the patient’s symptomatology is difficult to ascertain. This issue is further compounded by various angiographic limiting aspects, such as lesion eccentricity, vessel overlapping and tortuosity, degree of calcification, and diffuse reference vessel disease [24]. In this context, IVUS has the ability to provide complementary information to coronary angiography. In fact, the use of pre-intervention IVUS in coronary artery disease management has been reported to result in redirection of therapy in up to 40 % of patients [25].
Although fractional flow reserve (FFR) is currently being preferred as the investigational tool to assess the functional significance of an intermediate lesion [26–28], IVUS has an advantage in permitting precise quantification of anatomical distribution and morphology of a lesion; an important consideration in devising procedural strategy and device selection. Several studies have also reported reasonable correlation between structural data derived from IVUS with physiological parameters from FFR [29–34]. For example, earlier studies have identified IVUS-derived minimum lumen area (MLA) <4 mm2 as being haemodynamically significant when compared with FFR and SPECT imaging [29, 30]. Furthermore, using this MLA cut off, a long-term follow-up study reported a low event rate among patients who had their intervention deferred for an MLA ≥ 4 mm2 [31]. The latter studies, however, suggest a lower MLA cut-off value of 2.4–3.6 mm2 as being haemodynamically significant as compared with FFR [32–34]. This apparent discrepancy is not entirely surprising given a single MLA value is significantly influenced by multiple factors including involved vessel and its size, lesion location, and length, and the presence of plaque rupture [33]. Therefore a combination of IVUS-derived parameters, such as plaque burden, area stenosis, and lesion length also needs to be taken into consideration when assessing an intermediate lesion [22, 24, 35]. Illustration of basic IVUS measurement can be seen in Fig. 4.2.
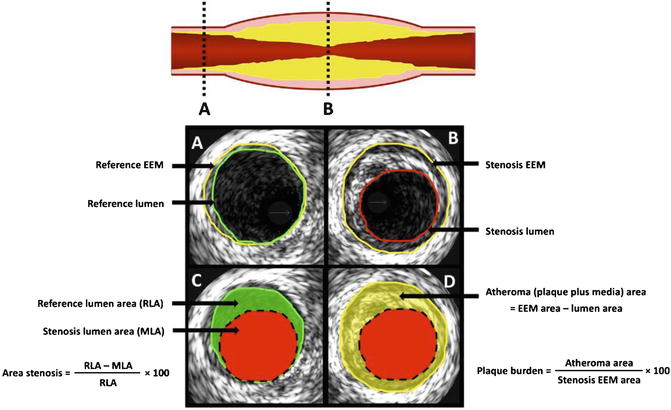

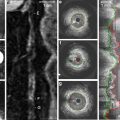
Fig. 4.2
Basic IVUS measurement. (a) The proximal reference segment. (b) Cross sectional image taken from the most stenotic segment. (c) The calculation of the area stenosis. (d) The IVUS quantification of plaque burden. Area stenosis is a measure of luminal stenosis relative to the normal reference segment. In contrast, plaque burden refers to the area within the EEM (external elastic membrane) which is occupied by atheroma (Reprinted from McDaniel, M.C., et al., Contemporary clinical applications of coronary intravascular ultrasound. JACC. Cardiovascular Interventions, 2011. 4(11): p. 1155–67. With permission from Elsevier)
Evaluations and Management of Left Main Coronary Artery Disease
Evaluation of Left Main Disease Severity
It has been well documented that quantifying angiographic left main disease severity especially that which involves the proximal segment is particularly challenging and is subject to significant interobserver variability [36, 37]. Three major anatomical factors which impair left main evaluation include aortic cusp opacification, short length of vessel trunk, and the presence of bifurcation or trifurcation at the distal segment [21]. Contrast streaming in the aortic cusp can obscure the ostium of the left main making angiographic evaluation problematic. On the other hand, the short segment of the left main shaft leaves little reference site for comparison. Also, the bifurcation into sub-branches at the distal end potentially conceals the distal left main. IVUS, in contrast, suffers not from the aforementioned limitation making it an investigation of choice when assessing left main lesion (Fig. 4.3).
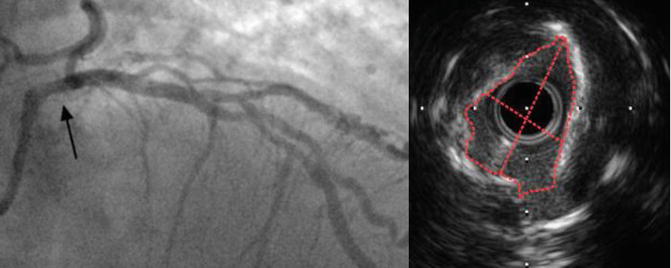

Fig. 4.3
Indeterminant ostial left main stenosis. This is the coronary angiogram picture taken from an 82-year-old man who presents with chest pain and strongly positive stress test (left). Black arrow demonstrates area of haziness involving the ostium of the left main coronary artery. This segment correlates with the IVUS image on the right which shows an eccentric, calcified left main lesion, with minimum lumen area (MLA) of 3.9 mm2. The patient was revascularised percutaneously and had a good outcome
In the case of angiographic intermediate left main stenosis, an IVUS minimum lumen diameter (MLD) of <2.8 mm or an MLA <6 mm2 suggest a physiologically significant stenosis and thus merits revascularisation [24, 26]. A study of 55 patients with moderate left main disease demonstrates that these cut-off values correlate well with physiologically significant lesion as assessed by FFR with a sensitivity of 93 % and a specificity of 98 % [38]. Additionally, a recent multicentre, prospective study showed that deferral of revascularisation among patients with left main MLA ≥ 6 mm2 carried a similar outcome with the revascularised group who has left main MLA < 6mm2 during long-term follow-up [39]. Another comparative study used an MLA cut-off value of 7.5 mm2 to determine whether a patient should undergo revascularisation or to have a deferral strategy [40]. It concluded that during the mean follow-up of 3.3 ± 2 years, there was no difference in major adverse cardiac event (MACE) between the two cohorts. Altogether, in conjunction with clinical information, it is reasonably safe to defer revascularisation in patients with MLA ≥ 7.5 mm2 and to consider revascularisation in patients with MLA < 6mm2. Patients with intermediate MLA value (6–7.5 mm2) would require further physiological assessment, for example with FFR [27]. Based on the available evidence, IVUS was given a class IIA indication for the assessment of angiographically indeterminant left main coronary artery disease by the recent joint American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI) guideline [41].
IVUS-Guided PCI for Unprotected Left Main Disease
Aside from its ability to provide further stratification of left main disease severity, IVUS also made a major contribution in the area of unprotected left main PCI. Patients with significant left main disease (≥50% stenosis) carry a high-risk mortality and morbidity from cardiovascular events considering the area of myocardium at risk should the flow in this vessel becomes compromised. To date, coronary artery bypass surgery remains the “gold standard” treatment for majority of significant left main disease especially in those with multivessel involvement. Nevertheless, the introduction of drug-eluting stent (DES) has ushered a new era of complex PCI, such as unprotected left main coronary artery disease and bifurcation lesion PCI due to its low rate of restenosis [42]. The use of pre-intervention IVUS in the left main PCI permits a detailed assessment of the vessel anatomy, the size of the vessel, and to determine the extent of ostial involvement at the daughter sub-branches [26, 43]. This information helps the operator to optimise stent selection and provide complete disease coverage. IVUS also assists in ensuring adequate stent expansion and apposition; important risk factors for restenosis and stent thrombosis [44–46]. Finally, IVUS also reveals the extent of calcification. This information will be useful in deciding whether to employ debulking strategy with rotational atherectomy to facilitate the stent placement [47–49].
The role of IVUS-guided strategy for unprotected left main PCI, however, remains a controversial issue given the conflicting data in the literature [46]. Nonetheless, several recent reports provide clinical data favouring its routine use. In a large Korean registry study [50], the use of IVUS-guided left main PCI was associated with a 3 year reduction in mortality when compared with angiographic guidance PCI. This benefit is particularly observed among the 145 matched pairs of patients who receive DES (4.7 % vs. 16 %, p = 0.048). Moreover, a prospective, randomised, non-inferior study comparing PCI vs. CABG for left main disease (PRECOMBAT trial) reported a low event rate in the PCI group and comparable outcome with the CABG group (8.7 % vs. 6.7 %, p = 0.02 for non-inferiority) [51]. In this multicentre trial, IVUS was extensively used. Some of this clinical benefit may be derived from IVUS ability to determine the extent of disease burden and lesion complexity thus assisting the operator with the appropriate procedure strategy. For instance, the MLA of the confluent zone between the distal segment of left main coronary artery and its daughter branches has been shown to be an accurate indicator of bifurcation left main disease severity and a predictor of post-stent underexpansion and clinical outcome [43, 52]. Another study then demonstrated that among patients who undergo bifurcation left main PCI, those with post-stenting underexpansion have significantly lower MACE-free survival rate in 2 years when compared with their counterpart [53]. Taken together, IVUS should be considered to guide unprotected left main PCI especially when it involves the bifurcation point.
IVUS and PCI
Refinement of PCI Technique and Practice
IVUS has been instrumental in helping us to understand the arterial responses to different coronary interventional modality [54–57] and has assisted in the improvement of technical details of the devices application and their manufacturing. For instance, IVUS has given a significant insight into the different mechanism of restenosis post-intervention. It discovers that whilst a combination of elastic recoil and concentric remodelling and to a lesser role, local intimal hyperplasia account for the mechanism of restenosis in post-balloon angioplasty [58–60], neointimal hyperplasia is the sole mechanism of late lumen loss in the stented artery receiving bare metal stent (BMS) [61, 62]. These observations were extremely valuable in guiding the development of management strategy for in stent restenosis, which ultimately leads to the development of DES. IVUS has also revolutionised the technique of stent deployment and post-stent medication regime as it is practised today. Following the enthusiasm with the introduction of stents, clinicians began to notice the issue with stent implantation in the form of subacute thrombosis [63]. This issue was further compounded by major bleeding issue due to intensive use of oral anticoagulation regime in order to reduce the incidence of stent thrombosis. IVUS then began to identify the association between subacute stent thrombosis and stent deployment issue, such as inadequate stent expansion and incomplete stent apposition (ISA) [64]. Inadequate stent expansion occurs when part of the stent is insufficiently expanded when compared to the proximal and distal reference site. Incomplete stent apposition, in contrast, refers to a lack of contact between the stent struts and the underlying arterial wall. Based on this observation, Colombo et al. developed post-stent deployment high pressure technique [65]. This strategy not only led to the reduction of stent thrombosis incidence but also allowed oral anticoagulation to be replaced with dual antiplatelet therapy thus reducing the length of hospital stay and vascular complication rate.
IVUS and Bare Metal Stent PCI
The benefit of IVUS in coronary intervention was initially demonstrated in the era of balloon angioplasty. Randomised and non-randomised IVUS trials demonstrated that IVUS-guided strategy resulted in the improvement of final lumen diameter and caused significant reduction in clinically driven reintervention rate when compared with the angiography group [66, 67]. Another randomised trial of 254 patients (BEST trial) then reported a similar clinical outcome between IVUS-guided balloon angioplasty (with provisional stenting) vs. routine angiography-guided stenting [68]. It is however worth noting that in this trial, there is a high crossover rate (44 %) in the balloon angioplasty group to stenting. Taken together, the use of IVUS is effective in optimising final balloon angiographic and clinical outcome. This approach, however, was viewed as time consuming and cost-ineffective especially as the stent profile continued to improve and its price continued to decline. Thus, routine stenting now is the preferred strategy above balloon angioplasty with provisional stenting [63].
The benefit of IVUS-guided strategy in BMS PCI has been examined in both observational [69–71] and randomised trials [72–75]. IVUS can be used to perform pre-interventional evaluation of the extent of lumen obstruction and the mechanism of obstruction (e.g. thrombus or calcification). This in turn allows for each management strategy to be individualised and tailored according to patient’s clinical baseline status. Post-intervention, IVUS may assist with optimising procedural outcome, such as assessment of stent expansion and stent strut apposition, detection of PCI complication (e,g., dissection, plaque shift), and identification of residual stenosis and stent fracture. Of these, inadequate stent expansion has been associated consistently with target vessel failure resulting from either in stent restenosis [71, 76–78] or stent thrombosis [79, 80]. Despite its practical use and demonstrable benefit by case control studies, the data regarding IVUS clinical benefit from randomised trials are conflicting. The TULIP study, a randomised controlled trial involving 144 patients with long disease segment (>20 mm) demonstrated a significant improvement in both angiographic and clinical end points in the IVUS-guided group when compared to the angiographic directed group [74]. Another trial, AVID, however reported a slight different outcome. AVID randomised 800 patients into IVUS vs. angiography-guided intervention with total follow-up of 12 months. Indeed, AVID is the largest randomised trial evaluating the benefit of IVUS in BMS PCI. In this trial, IVUS-guided strategy resulted in the improvement of post-procedural minimum stent area (a marker of adequate stent expansion) but did not result in the reduction of clinical end point (death/myocardial infarction/target lesion revascularisation) except in the subgroup with moderate size vessel (2.5–3.5 mm) and in saphenous vein graft PCI [72]. In this subgroup, the reduction in clinical end point is mainly driven by target lesion revascularisation. Thus the main clinical benefit of IVUS, based on these trials is the reduction of target vessel failure without added mortality benefit. These findings were further replicated in a meta-analysis from seven randomised trials involving 2,193 patients [81]. It concludes that IVUS guidance was associated with a significantly larger post-procedure angiographic MLD, lower rate of 6-month angiographic restenosis, a reduction in the revascularisation rate, and overall MACEs. However, no significant effect was seen for myocardial infarction or mortality. Overall, the efficacy of IVUS in optimising procedural outcome and reducing restenosis rate in BMS PCI is unquestionable, nonetheless its routine use in clinical practice still needs to be weighed against a number of factors, including cost, time, availability, and the operators skill, to accurately acquire and interpret the images.
IVUS and Drug-Eluting Stent PCI
The arrival of DES opened a new chapter in the world of interventional cardiology. DES had been shown to significantly reduce the risk of restenosis to less than 10 % [44, 82–85] when compared to BMS. It does this by inhibiting neointimal proliferation through its drug coating [86, 87]. As a result, operators began to put less attention on achieving optimal final procedural outcome as in the days of BMS [65]. However, the discovery of increasing incidence of stent thrombosis, especially very late stent thrombosis in the patients receiving DES has resulted in renewed interest in the use of IVUS to direct therapy.
Stent thrombosis, despite its rare occurrence (annual risk ~0.5%), carries a significant mortality and morbidity [88]. The overall prognosis is poor with 10–30% mortality rate. Several precipitating factors have been implicated to cause stent thrombosis and these essentially can be categorised as patient factors, lesion characteristics, device factors, and procedural factors. IVUS has provided significant insights into the morphologic pattern and possible causes of stent thrombosis following DES implantation, specifically stent underexpansion and incomplete stent apposition. Fuji et al. conducted a retrospective analysis on 15 patients who developed stent thrombosis following successful DES implantation [89]. They reported that lesions leading to stent thrombosis had more stent underexpansion, smaller minimum stent area, and residual edge stenosis. The strong association between IVUS detected stent underexpansion and stent thrombosis was also observed and well established in various other DES trials [90–95] and BMS trials [79, 80]. The role of incomplete stent apposition in causing stent thrombosis, on the other hand, is still controversial. Firstly, incomplete stent apposition is common, occurring in 10–20% of DES and can be acute or late [96]. Whilst acute ISA is procedural related, late ISA may be due to a combination of positive vessel remodelling (i.e. vessel expansion), intimal hyperplasia, or dissolved thrombus or plaque which led to a gap between vessel wall and stent [97]. Secondly, there is a discrepancy of observation among various trials regarding the link between ISA and stent thrombosis. In a case control study by Cook et al., a significantly high rate of ISA was noted in the very late stent thrombosis patients as compared to the DES control group [98]. This association was observed in another observational study [99] but not identified in follow-up studies of large randomised DES trials [83, 100–103]. In a recent sub-analysis of a randomised DES trial, Cook et al. reported that very late stent thrombosis and MACE occurs more frequently in patients with ISA than without ISA [104]. In this study, there was no difference in mortality observed.
Based on these IVUS observations, several trials have been conducted to assess the clinical benefit of IVUS-directed therapy in DES PCI. A study by Roy et al. compared 1-year clinical outcomes in 884 patients who underwent IVUS-guided PCI with a propensity-matched cohort of angiographically guided patients [105]. IVUS-directed therapy was found to be associated with lower incidence of stent thrombosis at 30 days (0.5% vs. 1.4%, p = 0.05) and 1 year (0.7% vs. 2.0%, p = 0.01) with no difference in the rates of myocardial infarction or death. Claessen et al. subsequently reported that IVUS guidance strategy resulted in significant reduction of early, medium-term, and long-term clinical outcome in a large registry study [106]. This benefit is mostly driven by reduction in the numbers of myocardial infarction. In another large observational study involving 8,371 patients, IVUS-guided DES PCI was also found to be associated with a 3-year reduction in mortality (HR 0.46; 95% CI 0.33–0.66, p < 0.001) [107]. Despite all these demonstrable benefits, to date there is still a lack of information regarding clinical outcome from IVUS-randomised trials. Indeed, there is only one randomised trial assessing the efficacy of IVUS use in DES implantation. AVIO trial randomised 284 patients with complex lesion (e.g. small and diffuse vessel disease, bifurcation lesion, chronic total occlusion) into IVUS-guided or angiography-guided arms [108]. Optimal stent expansion is defined as achieving ≥70% of the cross sectional area of the post-dilation balloon. The preliminary data from 9-month follow-up indicated that IVUS-guided strategy resulted in larger post-procedural MLD but no difference was observed in the rate of MACE. On balance, there is no strong recommendation for routine IVUS-guided PCI at this stage. However, IVUS use should be considered in patients with high risk of stent thrombosis or in patients whereby the consequence of stent thrombosis is fatal, such as left main coronary artery disease. IVUS should also be considered for evaluation of in stent restenosis or stent thrombosis, especially when it is a recurring event.
Safety
Despite its clinical advantages over coronary angiogram, the widespread use of IVUS has been somewhat limited by its invasive nature. The safety of IVUS has been investigated extensively and yields low overall rate of complication [109–111]. The most common complication recorded is transient vessel spasm, which occurs in the order of 2% of IVUS procedure. Major IVUS complications, such as dissection and abrupt vessel closure are quite rare with <0.5% of all procedures [111]. This mainly occurs in the vessel that undergoes simultaneous PCI or in ACS patients as supposed to patients who just undergo diagnostic imaging. Importantly, IVUS has not been shown to result in accelerating disease progression [112, 113].
Imaging Atherosclerosis
The Quest of Vulnerable Plaque
In the last decade, IVUS technology has been constantly used in the research arena in an attempt to identify vulnerable plaque, which is the major substrate of acute coronary syndrome. Vulnerable plaque or thin-capped fibroatheroma (TCFA) is defined histologically as a plaque with thin fibrous cap (<65 μm) which is associated with a large necrotic core (often containing haemorrhage or calcification), reduced smooth muscle content, and a large number of infiltrative inflammatory cells, such as macrophages and activated T cells [114]. Several other pathological features have also been observed to be present in TCFA, such as positive remodelling (expansion of the external elastic membrane in response to plaque accumulation) [115] and abundant intraplaque vasa vasorum, indicating neoangiogenesis and active inflammation [116, 117]. Some of these TCFA features like positive remodelling are discoverable by standard greyscale IVUS; however, it falls short in its capability to characterise plaque composition which determine vulnerability. Greyscale IVUS is also limited due to its considerable post-processing to produce an image and reliance on visual inspection of acoustic reflections to determine plaque component.
In an effort to improve the detection of plaque composition, an IVUS capability called virtual histology-IVUS (VH-IVUS) was developed. VH-IVUS uses mathematical autoregressive spectral analysis of the radiofrequency backscatter data to generate a tissue map of the four plaque components: fibrous (green), fibrofatty (yellow green), necrotic core (red), and dense calcium (white) [118] (Fig. 4.4). An alternative algorithm to characterise plaque composition called integrated backscatter-IVUS (IB-IVUS) has also been developed. This algorithm uses fast Fourier transform analysis of the backscatter signal of a tissue volume to generate tissue colour map [119, 120]. VH-IVUS accuracy in detecting these plaque types has been validated in vivo with a predictive accuracy of 87.1, 87.1, 88.3, and 96.5 % for fibrous, fibrofatty, necrotic core, and dense calcium respectively [121]. VH-IVUS, however, is limited in its ability to visualise thrombus and may misclassify it as fibrous or fibrofatty plaque. Owing to its axial resolution (200 μm), VH-IVUS is also unable to identify the fibrous cap thickness of TCFA, which is <65 μm. As a result, VH-IVUS-derived TCFA (VH-TCFA) has been defined as confluent necrotic core-rich plaque (>10%) without evidence of overlying fibrous tissue on three consecutive frames with the arc of the necrotic core in contact with the lumen for 36° along lumen circumference [122]. It also must have percent atheroma of ≥40% [123]. Based on these criteria, investigators have found that VH-TCFA is more prevalent in the patients with acute coronary syndrome than in patients with chronic stable angina, making it a reasonable surrogate for vulnerable plaque [124, 125].
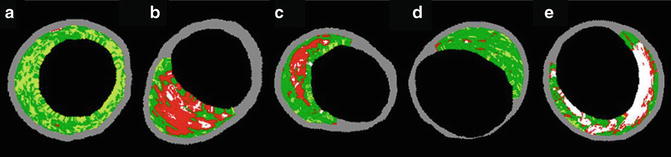

Fig. 4.4
Lesion type classification by VH-IVUS. Based on the VH-IVUS algorithm, coronary lesion, or plaque can be characterised according to the combination of different tissue colour map (fibrous, green; fibrofatty, yellow green; necrotic core, red; dense calcium, white). (a) Pathological intimal thickening. (b) VH-IVUS-derived thin-capped fibroatheroma. (c) Thick-capped fibroatheroma. (d) Fibrotic plaque. (e) Fibrocalcific plaque (Reprinted from Kubo, T., et al., The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterisation. Journal of the American College of Cardiology, 2010. 55(15): p. 1590–7. With permission from Elsevier)
Information derived from both IVUS and VH-IVUS have been critical in providing us with information to help predicting individual future coronary event. Yamagishi et al. analysed 106 patients with angiographic minimal coronary artery disease with baseline conventional IVUS [126]. At follow-up, plaques that resulted in acute coronary syndrome were found to exhibit higher baseline plaque volume, eccentric in its distribution, and manifest echolucent zone characteristics. Another study which analysed the impact of baseline plaque composition on future coronary event is PROSPECT trial (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) [127]. PROSPECT is the first prospective study utilising three imaging modality: coronary angiogram, conventional IVUS, and VH-IVUS to assess the natural history of vulnerable plaque. In this study, 697 patients with acute coronary syndrome underwent three vessel IVUS and VH-IVUS post-PCI with primary end point of MACE (death/cardiac arrest/myocardial infarction/rehospitalisation). After a median follow-up of 3.4 years, 20.4 % of patients experience MACE. Events were adjudicated to be related to culprit lesions in 12.9 % of patients and to non-culprit lesions in 11.6 %. Predictors for MACE in non-culprit lesion were identified to be diabetes, IVUS plaque burden ≥70%, MLA ≤ 4mm2, and the presence of VH-TCFA. In the absence of VH-TCFA, the event rate in the non-culprit lesion was reduced to 1.3–1.9% in 3 years depending on the presence of other risk factors. In another serial VH-IVUS study by Kubo et al., VH-TCFA may stabilise, remain unchanged, or even evolve in a new territory suggesting that the natural history of vulnerable plaque is a dynamic process [128]. Altogether, the combination of conventional IVUS and VH-IVUS could potentially provide a prognostic tool to predict a lesion with potential for coronary event, even in artery with angiographically minimal disease.
Besides VH-IVUS, there are several other IVUS-based technologies that have been developed in order to identify certain pathological features associated with vulnerable plaque. Some examples include IVUS elastography/palpography [129–131] and contrast-enhanced IVUS (CE-IVUS) [132–134]. The former attempts to identify the plaque vulnerability by analysing the mechanical strain property of the arterial wall [135]. The differences in tissue deformity potentially allow differentiation of various plaque components. On the other hand, CE-IVUS uses microbubble contrast agent in order to quantify vasa vasorum density and plaque perfusion, a feature of inflammation, which is one of the signatures of vulnerable plaque [134, 136]. This technology, however, remains an experimental tool at this stage and has not been established as a valid surrogate clinical end point.
Evaluation of Atherosclerotic Plaque Progression/Regression
Plaque progression, as measured by serial IVUS has been associated with an increased risk of future cardiac event [137, 138]. As a result, IVUS-measured atheroma change has been used extensively in randomised clinical trials as a surrogate clinical end point of various novel [139–144] or existing cardiovascular medication [145–151]. The use of IVUS as a surrogate end point allows trials to be conducted in a shorter time frame with smaller number of participants thus expediting the process of drug development. Among these trials, IVUS has provided especially a significant insight into the impact of statin therapy on the natural history of atheroma. The use of intensive statin therapy is not only shown to halt disease progression but also causes disease regression [145, 148]. Furthermore, VH-IVUS has extended this observation by demonstrating that statin therapy also results in the change of plaque composition [150, 152]. Altogether, these trials demonstrate that pharmacological therapy has the potentials to halt, reverse, and alter the course of the natural history of atheroma progression.
Future Perspectives
IVUS has made a major leap in imaging atherosclerosis with the continual expansion of its new capabilities. In the near future, we will see a more IVUS synergism with other imaging modality to improve tissue characterisation and arterial imaging. A co-registration with optical coherence tomography (OCT), for example is already on the horizon [153]. OCT has a much higher spatial resolution (10–20 μm) compared to conventional IVUS [154]. Owing to this, OCT is excellent in visualising the thin cap of TCFA and stent strut coverage. Some of its tissue characterisation includes red blood cell-rich thrombus, platelet-rich thrombus, and macrophages infiltration [155].
Recently, a combination catheter of IVUS and near-infrared spectroscopic (NIRS) imaging was developed and performed in vivo [156, 157]. NIRS provides information of plaque composition by analysing the pattern of near-infrared light absorbance by different tissue [158]. It is superior to IVUS in terms of its ability to detect the presence of cholesterol crystals which is abundant in the necrotic cores [159]. It, however, does not provide structural information. Thus the combination of these two imaging modalities would provide a superior, structural, and compositional information. Ultimately, the optimum intravascular imaging of coronary artery would combine the spectroscopic capabilities of NIRS, the near-field resolution of OCT, and the tissue penetration ability of IVUS.
Another exciting area from IVUS research is the use of IVUS to assess the relationship between segmental coronary endothelial function and regional plaque burden. It has been well established that endothelial dysfunction, as assessed by the vasodilator responses to different endothelial-dependent stimuli is associated with an increased risk of future cardiovascular event [160–162]. Recently, we published a data from our sets of experiment that looked at the relationship between plaque burden and endothelial function as a plausible explanation for the association of this phenomenon with adverse clinical events [163]. We utilised IVUS to evaluate the vasodilator properties of the study artery to the endothelial-dependent stimulus, intracoronary salbutamol. The study showed a strong relationship between in vivo segmental human coronary endothelial-dependent macrovascular and microvascular function with associated underlying atheroma burden. We have also undertaken a program to evaluate the impact of this dynamic process on atherosclerotic plaque progression (http://www.anzctr.org.au/ACTRN12612000594820.aspx). The impact of this dynamic process and its relationship to plaque burden and/or progression on clinical outcome is yet to be defined.
Summary
The role of IVUS in the world of interventional cardiology continues to evolve in parallel with the refinement of PCI technique, devices, and approach. IVUS has played an important role in helping us to understand the different arterial response to various interventional approaches as well as guiding the clinician with the device selection and strategy. Its role is even more paramount in the area of complex PCI, such as left main or bifurcation disease, whereby the consequences of complication could be severe. Advances in IVUS, such as VH-IVUS and IVUS with NIRS capability, also permit a more optimal characterisation of atherosclerosis plaque. This provides not only a significant insight into the natural history of atherosclerosis but also an opportunity to evaluate the impact of different pharmacological therapy on modifying the disease progression. Finally, large prospective clinical trials are needed to assess the clinical benefit of emerging novel IVUS technology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree