Vascular Abnormalities
Prachi Dubey
Aly H. Abayazeed
Sathish Kumar Dundamadappa
QUESTIONS
1 A 41-year-old female with no prior medical history presents with new-onset seizure. Following are the key images from an MRI.
|
1a Which of the following entities is the most likely explanation for these lesions?
A. CNS vasculitis
B. Progressive multifocal leukoencephalopathy
C. Lymphoma in immunocompetent individuals
D. Multiple sclerosis
View Answer
1a Answer A. CNS vasculitis. Presence of peripheral cortical/subcortical signal changes with presence of hemosiderin staining in the left frontal lobe, is most compatible with CNS vasculitis. The cortical extension is atypical with PML. Lymphoma can be multifocal and hemorrhagic in immunocompromised but very unlikely morphology in immunocompetent patients. Rare variant of tumefactive demyelination or ADEM can have this appearance; fulminant forms may hemorrhage but will not be a likely or usual consideration.
1b Which of the following tests is most useful in confirmation of the diagnosis of primary angiitis of the CNS?
A. Conventional cerebral angiography
B. MR angiography with contrast
C. High-resolution vessel wall imaging
D. Surgical biopsy
View Answer
1b Answer D. Surgical biopsy. The peripheral small-vessel involvement lowers the yield of diagnostic imaging in confirming the diagnosis. Often, these studies are normal despite path-proven primary CNS angiitis changes seen on cortical and meningeal biopsy.
|
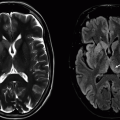
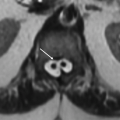
Imaging Findings: Axial FLAIR (A and C) showing scattered cortical/subcortical white matter lesions with left frontal lobe focus showing small hemosiderin staining (B).
Discussion: Vasculitis is a broad term, which includes multiple diseases with a common feature of inflammation of the vessel wall involving arteries, veins, or both. It is responsible for about 5% of strokes in young patients.
Vasculitis involving the nervous system is classified into primary angiitis of the central nervous system (PACNS) and secondary vasculitis. Secondary vasculitis can be primary systemic vasculitis with CNS involvement or secondary vascular involvement with a systemic disease. Vasculitis-like picture can also be seen with recreation drug use and as long-term sequel of radiation.
Vasculitis is also subclassified based on the size of the smallest involved vessel as large-vessel vasculitis (defined as vessel larger than 2 mm in size, vessels including and proximal to M1/A1/P1), medium-vessel vasculitis (vessel size of 0.5 mm), and small-vessel vasculitis (vessel size of 0.2 to 0.3 mm, leptomeningeal and cortical vessels).
Common systemic vascular diseases involving CNS are giant cell arteritis (large vessel), Takayasu disease (large vessel), Wegener granulomatosis (small vessel), polyarteritis nodosa (medium vessel), and Churg-Strauss syndrome (small vessel). Sjögren syndrome, mixed connective tissue disorders, SLE, and neuro-Behçet’s are some of the systemic diseases with secondary vascular involvement.
PACNS aka primary CNS vasculitis is by definition confined to central or peripheral nervous system. This is rare disorder with peak incidence in 4th to 6th decades of life and commonly affecting small vessels and occasionally medium-sized vessels. Veins are affected less often. Histopathologically, there is focal or segmental inflammatory infiltrate accompanied by vessel wall necrosis with or without perivascular granuloma, myelin loss, and axonal degeneration. The disease is heterogeneous with varied and often nonspecific clinical presentation. Imaging is also nonspecific and reflects sequela of vessel abnormality (intervening areas of stenosis and dilation, “beaded appearance”), ischemia following luminal compromise, and hemorrhage following vessel wall disruption.
Imaging evaluation of the vasculitis should begin with MRI, evaluating the parenchymal effects. This should be followed by vascular imaging and if necessary biopsy.
The most common imaging abnormality in PACNS is bilateral, multiple, supratentorial white matter T2 hyperintensities. Basal ganglia and cortex can also be involved. Multiple infarcts in different vascular territories can be seen. Contrast enhancement of the lesions, meninges, and perivascular spaces can be seen. Mass-like lesions are seen in about 15% of cases. Parenchymal and subarachnoid hemorrhages often small and petechial can be present, best depicted by SWI images. DWI restriction can be seen in newer lesions in the background of older lesions.
Noninvasive vascular imaging is often negative in PACNS as small vessels are below the resolution of CTA/MRA. Catheter angiogram can be negative unless there is involvement of the medium-sized vessels. Meningeal or brain biopsy is the mainstay for diagnosis in small-vessel vasculitis. Given the heterogeneity of the disease, even biopsy can be false negative.
References: Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol 2009;66(6):704-709. doi:10.1001/archneurol.2009.76
Garg A. Vascular brain pathologies. Neuroimaging Clin N Am 2011;21(4):897-926. doi:10.1016/j. nic.2011.07.007
Hähnel S, ed. Inflammatory diseases of the brain, 2nd ed. 2013 ed. New York, NY: Springer, 2013.
Law M, Som PM, Naidich TP. Problem solving in neuroradiology: expert consult—online and print, 1st ed. Philadelphia, PA: Saunders, 2011.
Naidich TP, ed. Imaging of the brain. Expert Radiology Series. Philadelphia, PA: Saunders/Elsevier, 2013.
2 A 50-year-old male with neck pain and vertigo presents to the emergency department. Key images from an MRI are provided below:
|
2a What is the sequence type shown in Figure A?
A. T1w axial with contrast and blood suppression
B. T1w axial with fat saturation
C. T2w axial with fat saturation
D. T2w axial with contrast and blood suppression
View Answer
2a Answer B. T1w axial with fat saturation. The axial image shows T1 weighting (note low signal from CSF in cervical canal and intermediate signal from mucosal surface). There is also suppressed signal from subcutaneous fat. There is no contrast or blood suppression.
2b What is the most common imaging manifestation of the above diagnosis?
A. Arterial stenosis
B. Intimal flap
C. Intimal hematoma
D. Pseudoaneurysm
View Answer
2b Answer A. Arterial stenosis. The diagnosis here is vertebral artery dissection. Often on imaging, it manifests as an abrupt change in vessel caliber or tapering stenosis not accounted by atherosclerotic disease.
|
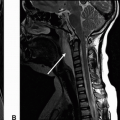
Imaging Findings: There is a crescenteric focus of T1 hyperintensity seen in the V2 segment of the right vertebral artery on axial T1 fat saturation without contrast (A). This corresponds to mural thickening and string-like luminal narrowing at this level on CTA (B). Note focal dilation just distal to the string-like narrowing seen on coronal CTA MIP (C).
Discussion: Vertebral artery dissection (VAD) is an important diagnostic consideration in patients with neck or head pain and vertigo/dizziness. It may also result in an ischemic stroke and should be carefully evaluated in the posterior circulation acute ischemic strokes. The causes include spontaneous, traumatic, iatrogenic neck manipulation, or having predispositions such as fibromuscular disease and collagen vascular disorders.
Clinically, these patients can present with neck pain and dizziness or vertigo. Additional neurologic deficits may be present if complication by a posterior circulation infarction or subarachnoid hemorrhage.
On imaging, CTA and MRA can be both used for noninvasive diagnosis. A T1 or T2 fat saturation sequence can be used to assess for intramural hematoma. Commonly, however, the VAD demonstrates vascular stenosis with vessel wall thickening, string sign, because of marked luminal narrowing or abrupt changes in vessel caliber. There may be vessel wall irregularity that can be better seen with high-resolution vessel wall imaging or conventional cerebral angiography, the latter considered the gold standard for VAD diagnosis.
Vertebral artery dissection itself can have a steno-occlusive or aneurysmal morphology. The aneurysmal pattern can have a focal or fusiform dilation proximal or distal to the stenotic segment. This has been most frequently found in the V4 segment and may be associated with subarachnoid hemorrhage. When the dissection progresses through the media into the subadventitial layer, it results in eccentric outer wall dilation, leading to a dissecting pseudoaneurysm. Management largely depends upon clinical symptoms, location, and morphology of the dissection. Anticoagulation can be used in patients with extradural dissection. However, it is contraindicated in patients who have hemorrhagic infarction or subarachnoid hemorrhage. Endovascular coil or surgical embolization of dissecting aneurysms can be performed.
References: Gottesman RF, Sharma P, Robinson KA, et al. Imaging characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist 2012;18(5):255-260.
Shin JH, Suh DC, Choi CG, et al. Vertebral artery dissection: spectrum of imaging findings with emphasis on angiography and correlation with clinical presentation. Radiographics 2000;20(6):1687-1696.
3 A 55-year-old male with sudden-onset right-sided weakness and altered mental status.
|
3a Given the noncontrast head CT findings, what is the next best study?
A. Digital subtraction angiography
B. CT angiography and CT perfusion
C. Brain MRI and MRI perfusion
D. No additional workup needed, only treatment
View Answer
3a Answer B. CT angiography and CT perfusion. There is hypoattenuation in the left basal ganglia region with loss of definition of left caudate and putamen (Fig. A). There is also a hyperdense vessel in the LMCA region, concerning for a thrombus (Fig. B). This is compatible with acute stroke and B is therefore the next step in workup.
3b Below are key images from the coronal CTA, CBF, CBV, and MTT maps (in that order) from CT angiography and CT perfusion. What is the most accurate diagnosis?
|
A. Left ICA paraclinoid/terminus occlusion with LMCA ischemic penumbra
B. Left ICA paraclinoid/terminus occlusion without an ischemic penumbra
C. Left ICA paraclinoid/terminus occlusion with chronic LMCA infarction
D. Left ICA paraclinoid/terminus occlusion without perfusion deficits
View Answer
3b Answer A. Left ICA paraclinoid/terminus occlusion with LMCA ischemic penumbra. The MTT and CBF maps show ischemic region in the LMCA distribution and low CBV in the basal ganglia region, suggesting a small core infarct with a mismatch perfusion defect compatible with an ischemic penumbra.
Imaging Findings: Figure 1. Axial noncontrast head CT demonstrates hyperdense left MCA sign because of intraluminal thrombus and cytotoxic edema/infarction in the left caudate and lentiform with loss of the normal gray-white matter differentiation.
Figure 2. Coronal MIP at the level of the carotid terminus from the CTA showing occlusion of the left carotid terminus and the left MCA. CBF, CBV, and MTT maps from the CT perfusion demonstrate a small area of core infarction (reduced CBV, which matches with severely reduced CBF, coded as dark blue on CBF map) in the left caudate and lentiform nuclei and large area of tissue at risk/penumbra (mismatched CBV/MTT) in the left frontal and parietal lobes in the vascular territory of the left MCA, which denotes patent distal pial collaterals supplying the ischemic tissue and potentially salvageable tissue.
Discussion: In cases of acute stroke, a noncontrast head CT is the first study performed to exclude intracranial hemorrhage and look for signs of intracranial ischemia. IV tPA is administered if the patient is within the therapeutic window for medical treatment and there is no intracranial hemorrhage. IA tPA can be administered in anterior circulation strokes up to 6 h from the time of onset.
It is the common practice to obtain CTA with or without CT perfusion studies in the setting of acute stroke to evaluate for possible intervention if the patient is within the therapeutic window (0 to 8 h in the anterior circulation). CTA will evaluate the site and extent of the vascular pathology (in situ thrombus, emboli, or dissection) and will show the status of the vessels proximal and distal to the site of occlusion and the status of collateral blood vessels. Core infarction (site of irreversible loss of function) will show matched CBF and CBV defects. Areas of tissue at risk (site of potentially salvageable neurons or penumbra) will show decreased CBF in mL/100 g brain tissue/min, maintained CBV in mL/100 g brain tissue (open collaterals), and prolonged MTT in seconds (it takes longer for blood to flow around the collateral vessels). Of note 4D CTA or dynamic CT angiography has been shown recently to be superior to spCTA (single-phase CTA) in evaluating the degree of vascular occlusion in ischemic lesions, tandem vascular lesions, the status of the delayed collateral circulation, and the spot sign in case of intracranial hemorrhage, a sign that is associated with increased risk of hematoma expansion. DWI-MRI is very sensitive towards accurate detection of infarct core. However due to MRI availability related limitations, CT remains the mainstay for work-up in many institutions.
References: Allen LM, Hasso AN, Handwerker J, et al. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics 2012;32(5):1285-1297.
Kortman HGJ, Smit EJ, Oei MTH, et al. 4D-CTA in neurovascular disease: a review. AJNR Am J Neuroradiol 2015;36:1026-1033; originally published online on October 29, 2014, doi:10.3174/ajnr.A4162
Lui TW, Tang ER, Allmendinger AM, et al. Evaluation of CT perfusion in the setting of cerebral ischemia: patterns and pitfalls. AJNR Am J Neuroradiol 2010;31:1552-1563; originally published online on February 25, 2010, doi:10.3174/ajnr.A2026
4 Images A, B and C were obtained during the initial presentation and Image D was obtained at 2 month follow up.
|
4a Based on the above images, what is the most likely symptom in this 35-year-old otherwise healthy female?
A. Thunderclap headache
B. Positional vertigo
C. Painful visual loss
D. Gait disturbances
View Answer
4a Answer A. Thunderclap headache. A classical symptom associated with RCVS. Note that this symptom is also seen with aneurysm rupture making it a challenging diagnosis based on clinical picture alone.
4b Which of the following is the key feature in reversible cerebral vasoconstriction syndrome (RCVS)?
A. Lack of involvement of medium-/large-sized vessels
B. Complete resolution of symptoms but persistent imaging findings
C. Treatment by magnesium is crucial
D. Complete resolution of symptoms and imaging findings within 3 months
View Answer
4b Answer D. Complete resolution of symptoms and imaging findings within 3 months.
|
Imaging Findings: There is focal left superior parietal sulcal nonsuppression of CSF on FLAIR suggesting trace subarachnoid hemorrhage (A). There are areas of focal segmental narrowing seen on MRA indicated by arrows (B and C) with involvement of right ACA A1 segment (C). These findings in the current clinical setting and age group are most likely to represent RCVS. Two-month follow-up image from MRA in the same patient shows complete resolution of previously seen findings (D).
Discussion: Reversible cerebral vasoconstriction syndrome (RCVS) refers to a group of disorders characterized by severe headache (often thunderclap type and recurrent in first week or two of the disease) with or without other neurologic symptoms and focal and segmental noninflammatory constrictions in multiple intracranial arteries that resolve spontaneously within 1 to 3 months. It has uniphasic course without new symptoms after 1 month of onset. “Thunderclap headache” refers to acute-onset extreme headache that reaches severity in less than a minute.
A wide range of age group can be involved, the peak at about 40 years of age, with female preponderance. Less than half of the cases are spontaneous. In nearly 60% of cases, precipitating factor is present, the most common being postpartum state and exposure to several recreational drugs (cannabis, cocaine, ecstasy, LSD, amphetamine derivatives) as well medications (nasal decongestants, SSRIs, etc.) many of which are vasoactive. Binge alcohol consumption is also reported trigger. Neck arterial dissection and carotid endarterectomy are also known triggers.
Exact pathophysiology of the disease is not known. This is believed to result from transient disturbance in maintenance of vascular tone and sympathetic overreactivity. Association with reversible leukoencephalopathy (PRES) and brain edema suggests the possibility of endothelial dysfunction as a contributory factor. The disturbance is hypothesized to start with smaller arteries and then progress to medium- and large-sized arteries over the next couple of weeks. Hence, RCVS is a dynamic process and clinicoradiologic features depend on the time course of the disease. PRES on the other hand has a propensity for medium- and small-sized vessels.
Given the dynamic nature of the disease, in early stages, imaging can be negative and “headache” can be the only symptom. A short-term repeat imaging is recommended. If there is associated neck pain, neck vascular imaging is suggested to look for dissection. Transcranial Doppler can be useful in monitoring cerebral vasoconstriction. Biopsy is not recommended for RCVS as this is a noninflammatory process and should only be done if there is a strong suspicion for true vasculitis. The prognosis of the disease is determined by the presence and extent of stroke; however, usually, this disease has a self-limiting benign course and responds to calcium channel blockers in the acute phase.
Differential diagnoses:
Aneurysmal SAH: Distribution of hemorrhage is often typical and generally more central. Vasospasm is generally limited to the areas of hemorrhage and the extent somewhat proportionate to the amount of hemorrhage. In RCVS, the stenoses are widespread and disproportionate to the small peripheral SAH. Unlike, SAH, headache of RCVS is short lived.
CNS vasculitis: Small peripheral hemorrhages can also be seen with vasculitis. In vasculitis, the onset of symptoms is more gradual. MRI generally shows presence of white matter lesions as well as infarcts at the time of presentation. Headaches are frequent with vasculitis but generally not thunderclap type.
References: Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 2012;11(10):906-917.
Ducros A, Boukobza M, Porcher R, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 2007;130(12):3091-3101.
5 A 45-year-old male with conjunctival chemosis and proptosis presents for initial evaluation. Key images from a CTA head are provided below.
|
5a What is a common etiology for the above changes?
A. Developmental in nature
B. Posttraumatic etiology
C. Postinflammatory changes
D. Related to radiation therapy
View Answer
5a Answer B. Posttraumatic etiology. A carotid cavernous fistula (CC fistula) is seen with aneurysmal dilation of the left supraclinoid ICA, engorged left cavernous ICA, and left superior ophthalmic vein. Trauma is a common etiologic factor leading to this condition.
5b What specific ocular abnormality is seen with the above diagnosis?
A. Orbital varix
B. Carotid cave aneurysm
C. Engorgement of the superior ophthalmic vein
D. Retrobulbar hemorrhage
5c What is the most likely etiology for mass-like lesion in left cavernous sinus?
A. Lymphoma
B. Meningioma
C. Tolosa-Hunt syndrome
D. Arterialized cavernous sinus
View Answer
5c Answer D. Arterialized cavernous sinus. A distended arterially enhancing left cavernous sinus is seen in the setting of a dilated left superior ophthalmic vein compatible with a CC fistula.
|
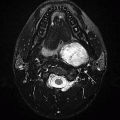
Imaging Findings: Enlarged and arterialized superior ophthalmic vein and vascular engorgement of left cavernous sinus seen on CTA source images (A to C). Catheter angiogram confirms CCF with venous congestion in orbit and in pterygoid venous plexus/masticator space (D).
Discussion: Carotid-cavernous fistula (CC fistula) is abnormal communication between the ICA and cavernous sinus shunting the carotid arterial blood into the cavernous venous system.
Carotid-cavernous sinus fistula (CCF) can be classified based on etiology (traumatic or spontaneous), rate of flow (high vs. low), or the angiographic architecture (direct, resulting from an ICA tear or indirect, which are due to meningeal branches from ICA and/or ECA shunting via a dural AV fistula).
The most commonly used calcification is by Barrow et al.:
Direct (type A fistula): Direct communication between internal carotid artery and cavernous sinus, usually high-flow fistula, commonly resulting from trauma. A smaller percentage (20%) can be spontaneous, resulting from rupture of either aneurysm or weakened ICA vessel wall.
Indirect CCF (types, B, C, and D): Dual arteriovenous fistulas, fed by meningeal branches of ICA (type B), external carotid artery (type C), or both (type D, the most common type of indirect CCF). These are more commonly low-flow fistulas, sequel of venous thrombosis.
Trauma to skull base is a common etiology for CCF. It can also be seen with ICA aneurysm rupture into the cavernous sinus. The indirect CCFs may result from revascularization of a prior cavernous sinus thrombosis. Clinically, these patients have ocular symptoms such as proptosis, conjunctival injection, chemosis, diplopia, or visual loss.
Imaging features on CTA or MRA include engorgement and arterial enhancement of the ipsilateral cavernous sinus and engorgement of the superior ophthalmic vein; bilateral cavernous sinus enlargement can also be seen due to intercavernous communications. T2-weighted MR images show abnormal flow voids within the cavernous sinus; abnormal hyperintense signal can be seen on time-of-flight MRA within the cavernous sinus.
Findings related to venous congestion are commonly seen in the orbits and include enlarged superior ophthalmic veins, enlargement of extraocular muscles, proptosis, and orbital edema. Intracranial venous congestion can present as engorged leptomeningeal and cortical veins and white matter T2 hyperintensity. One to two percent of patients can present with intracranial hemorrhage.
Conventional cerebral angiogram is the gold standard in identifying the exact site of communication, presence of dural feeders, and rate of flow.
Management depends on the type of fistula and symptoms. The direct CCF are typically high flow and have high risk for intracranial hemorrhage requiring timely intervention. Endovascular treatment with balloon, coil, or stent embolization can be performed. If needed, surgical ligation, cavernous sinus packing, or stereotactic radiosurgery can be considered. Conservative treatment is generally undertaken for indirect CCF, especially when the clinical symptoms are mild. Twenty to fifty percent of indirect CCFs heal spontaneously.
References: Gemmete JJ, Ansari SA, Gandhi D. Endovascular treatment of carotid cavernous fistulas. Neuroimaging Clin N Am 2009;19(2):241-255. doi:10.1016/j.nic.2009.01.006
Jindal G, Miller T, Raghavan P, et al. Imaging evaluation and treatment of vascular lesions at the skull base. Radiol Clin North Am 2017;55(1):151-166.
Lee JY, Jung C, Ihn YK, et al. Multidetector CT angiography in the diagnosis and classification of carotid-cavernous fistula. Clin Radiol 2016;71(1):e64-e71.
A. Developmental venous anomaly (DVA)
B. Cavernous malformation
C. Arteriovenous malformation (AVM)
D. Dural arteriovenous fistula (DAVF)
View Answer
6a Answer C. Arteriovenous malformation (AVM). There is large tangle of vessels with distended vein of Galen and a proliferative nidus seen on axial T2w MR image.
6b Which of the following is one of the key differentiating features between AVM and dural arteriovenous fistula (DAVF)?
A. Presence of intraparenchymal nidus
B. Large-vessel arterial feeder
C. Deep venous drainage
D. Size
View Answer
6b Answer A. Presence of intraparenchymal nidus. This is the key feature seen with AVM but not with DAVF. Both have arterial feeders shunting into the venous system. Extent of abnormality can be variable depending on the severity of the shunt.
|
Imaging Findings: Contrast-enhanced CT shows prominent tangle of vessels in the left parieto-occipital region with a distended vein of Galen (A). There is a diffuse or proliferative intraparenchymal nidus seen on axial T2w MRI (B).
Discussion: Brain arteriovenous malformations (bAVMs) are abnormal communications between arterial feeders, specifically pial, supplying the brain tissue and venous drainage pathways. This results in shunting of arterial blood directly into the venous pathways without an intervening normal capillary network. For the diagnosis of AVMs, two characteristic features are important: first, presence of an intraparenchymal nidus and, second, presence of early venous drainage seen on dynamic imaging such as conventional catheter angiogram.
Overall, these are one of rare causes for intraparenchymal hemorrhage and stroke. However, in younger individuals, this is the most common cause of intracranial hemorrhage. It can be isolated in nature or occur in association with syndromes such as Osler-Weber-Rendu (hereditary hemorrhagic telangiectasia [HHT]) or Wyburn–Mason syndrome.
Clinically, these patients can present with seizures, headache, focal neurologic deficits, and hemorrhage. On imaging, key features are presence of a parenchymal nidus, which may be compact or be diffuse with interspersed brain tissue, presence of arterial feeders, and early venous drainage. These lesions can be further classified using the Spetzler-Martin grading system designed to predict the risk of surgery.
Size of nidus: small <3 mm (1 point), medium 3-6 mm (2 points), and large (>6 mm) (3 points)
Venous drainage: superficial (0 points) or deep venous system (1 point)
Eloquence of adjacent brain: noneloquent (0 points) versus eloquent (1 point). This is determined by importance of the brain structure in terms of functional significance, such as sensorimotor cortex, visual cortex, language, thalamus/hypothalamus, brainstem, internal capsule, cerebellar peduncles, and deep cerebellar nuclei, which are all considered eloquent.
The grade is defined as sum of points in each category. Therefore, the highest possible grade is V (3+1+1). These lesions are deemed high risk with significant surgical morbidity and mortality. Management can be medical management with or without intervention such as surgery, endovascular embolization, or stereotactic radiosurgery.
References: Geibprasert S, Pongpech S, Jiarakongmun P, et al. Radiologic assessment of brain arteriovenous malformations: what clinicians need to know. Radiographics 2010;30(2):483-501.
Speizler RF, Martin NA. A proposed grading system for arteriovenous malformations. 1986. J Neurosurg 2008;108(1):186-193.
7 A 25-year-old female with recurrent transient ischemic attacks presents for an MRI/MRA. Key images are shown below.
|
7a Based on these images, what is the most likely explanation for the abnormality shown in Figures A and B?
A. Abnormal oxygenation
B. Subarachnoid hemorrhage
C. Meningitis
D. Pial vascular engorgement
View Answer
7a Answer D. Pial vascular engorgement. This condition is compatible with moyamoya syndrome, which is typically associated with extensive collateralization and related pial vascular engorgement. The sulcal nonsuppression of CSF shown in this case is likely resulting for vascular congestion. On contrast-enhanced MRI, this may manifest as pial enhancement, namely, the “Ivy sign.”
7b Which of the following is an accepted management strategy for this condition?
A. Vascular stenting
B. Endovascular aneurysm coil embolization
C. ECA (superficial temporal artery branch) to MCA anastomoses
D. Intravenous antibiotics
View Answer
7b Answer C. ECA (superficial temporal artery branch) to MCA anastomoses.
|
Imaging Findings: Axial FLAIR images showing sulcal nonsuppression of CSF (A and B) because of pial vascular engorgement in the setting of hypoplasia of M1-MCA bilaterally as noted on axial T2 weighted image (C) through the sylvian fissures bilaterally. 3D volume-rendered reformats from MR angiogram (D and E) confirm absent M1-MCA and A1-ACA bilaterally. These findings are consistent with moyamoya disease.
Discussion: Moyamoya disease is a progressive cerebrovascular pathology of unclear etiology. It is commonly seen in Asian population and has a female predilection. The disease has a bimodal distribution, seen in 1st and 3rd to 4th decade of life. Moyamoya-like vascular lesions can be seen with conditions such as sickle cell disease, NF1, radiation exposure, HCV infection, and cryoglobulinemia, among other numerous etiologies. In the presence of an etiologic factor, the condition is referred to as moyamoya syndrome.
Clinically, these patients can present with headaches, vertigo, transient ischemic attacks, stroke, hemorrhage, and seizures. Imaging findings include progressive nonatherosclerotic stenosis or occlusion of terminal portion of the ICA and proximal portions of ACA and MCA with extensive collateral formation, leading to the “puff of smoke” appearance on conventional angiogram. These collateral vessels are prone to both thrombotic occlusion and hemorrhage because of microaneurysm formation. FLAIR and postcontrast T1w sequences can show pial vascular congestion-related hyperintensity, namely, the “Ivy sign.”
Management can range from observation in mild cases to medial management with antiplatelet medication and surgery for severe cases. Surgery involved direct and indirect anastomosis using superficial temporal artery and middle cerebral artery (direct) or through directly placing the vascularized tissue on the cortex, such as with encephaloduroarteriosynangiosis (EDAS) or encephaloduroarteriomyosynangiosis (EDAMS). Prognosis is variable ranging from slow progression with rare ischemic events to rapid neurologic deterioration with infarction and hemorrhage.
References: Tarasów E, Kulakowska A, Lukasiewicz A, et al. Moyamoya disease: diagnostic imaging. Pol J Radiol 2011;76(1):73-79.
Yoon HK, Shin HJ, Chang YW. “Ivy sign” in childhood moyamoya disease: depiction on FLAIR and contrast-enhanced T1-weighted MR images. Radiology 2002;223(2):384-389.
8 A 35-year-old female with severe headache presents to the ER for further assessment. Key images from a CT and MRI are provided below.
|
8a What is a possible relevant clinical history in this patient?
A. High-dose aspirin use
B. Oral contraceptive use
C. Cocaine abuse
D. Uncontrolled hypertension
View Answer
8a Answer B. Oral contraceptive use. The images show bilateral subarachnoid and small focus of parenchymal hemorrhage in superior convexities. This is seen in the setting of thrombus within the superior sagittal sinus seen on the CTA. Oral contraceptive use predisposes to sinus thrombosis.
8b Which of the following venous structures is part of deep venous drainage?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree