4 Vascular Applications of Ultrasound Contrast Agents
Types of Ultrasound Contrast Agents
For more than two decades intravenously administered agitated saline has been utilized for “bubble study” echocardiography examinations.1 Hand agitation of saline results in the formation of microbubbles. After injection into a peripheral vein, the microbubbles increase the reflectivity of the blood in the right heart (and right-to-left intracardiac shunts when present), and this can easily be detected with gray-scale and Doppler US modes. However, agitated saline cannot be used to evaluate the left heart chambers or the systemic circulation because the microbubbles do not normally cross the pulmonary circulation and reach the left cardiac chambers.
Commercially available UCAs are nontoxic, have microbubbles that are small enough to traverse the pulmonary capillary beds (i.e., <8 µm in size) but large enough to reflect US signals. They are also stable enough to provide multiple recirculations, which results in several minutes of contrast enhancement after an IV bolus injection. Most UCA microbubbles contain heavy gases (e.g., sulfur hexafluoride or SF6, or fluorocarbons) that improve microbubble longevity after administration.2 Microbubble stability is provided by the shell, which is most commonly composed of phospholipids, surfactants, and other compounds. Table 4-1 lists UCAs that are FDA approved in the United States and their approved indications, and Table 4-2 lists UCAs that are commercially available elsewhere in the world. Several reports have confirmed that when used appropriately UCAs have very acceptable safety profiles.3,4
TABLE 4-1 UCAs that are Approved by the U.S. Food and Drug Administration*
| Agent, Manufacturer | Approved Indication(s) |
|---|---|
| Optison™, GE Healthcare, Princeton, NJ | Left ventricular opacification / endocardial border definition (LVO/EDB) |
| Definity®, Lantheus Medical Imaging, N. Billerica, MA | LVO/EDB |
| Imagent®, IMCOR Pharmaceuticals, Inc., San Diego, CA | LVO/EDB (Imagent is not currently being marketed.) |
* This information is considered accurate as of June, 10, 2011. The status of ultrasound contrast agents in the United States is subject to change.
TABLE 4-2 UCAs that are Commercially Available Outside the United States*
| Agent, Manufacturer | Countries | Approved Indications |
|---|---|---|
| Optison™, GE Healthcare, Chalfont St. Giles, UK | European Union | Left ventricular opacification / endocardial border definition (LVO/EDB) |
| SonoVue®, Bracco Imaging S.p.A., Milan, Italy | European Union, Norway, Switzerland, China, Singapore, Hong Kong, South Korea, Iceland, India, Canada | LVO/EBD, breast, liver, portal vein, extracranial carotid, peripheral arteries (macrovascular, microvascular) Approved in Canada for LVO/EBD and diagnostic assessment of vessels |
| Definity®, Lantheus Medical Imaging, N. Billerica, MA | Canada, Mexico, Israel, New Zealand, India, Australia European Union, Korea, Singapore, United Arab Emirates | LVO/EBD, liver, kidney Approved in these countries only for LVO/EBD |
| Sonazoid®, Daiichi Pharmaceutical Co., Ltd, Tokyo, Japan (Manufactured and distributed in partnership with GE Healthcare) | Japan | Focal liver lesions |
* This information is considered accurate as of June, 10, 2011. The status of ultrasound contrast agents around the world is subject to change.
Vascular Agents
Vascular or “blood-pool” UCAs enhance Doppler flow signals by adding more and stronger acoustic scatterers in the blood.5 This results in improved detection of blood flow signals from vessels that are often difficult to assess (e.g., the intracranial vessels, renal arteries, and small capillaries within organs). When used with “contrast-specific” imaging modes (see later), UCAs also improve gray-scale US visualization of flowing blood and increase the echogenicity of contrast-containing tissues.
In general, after IV administration, vascular UCAs remain in the body’s vascular spaces. When the microbubbles break down or are ruptured, the components of the microbubble shell are metabolized or eliminated by the body and the gas is exhaled.2
Tissue-Specific and Targeted Agents
Tissue-specific UCAs differ from blood-pool UCAs in that these agents are designed to attach to or enter the cells of specific tissues. UCAs have been developed to target plaque and thrombus.6 By actively attaching to the particular target, the UCA increases the echogenicity of the surface. These microbubble agents may attach to fibrin, platelets, or other components of thrombi.7 The presence of thrombus associated with a plaque as determined by CEUS might alter patient management and therapy. Some tissue-specific UCAs can be used to improve the detection of blood flow, as well as enhance the echogenicity of targeted tissue.
Sonazoid (GE Healthcare, Oslo, Norway) contains microbubbles that are targeted to the reticuloendothelial system.8 This agent has been used to improve detection of liver lesions in humans and for lymphatic applications in animal models.9 Tissue-specific UCAs target specific types of tissues, and their behavior is predictable so they can be classified as molecular imaging agents.10
Therapeutic UCAs
Thrombus-binding UCAs are an example of such an application. A microbubble bound to thrombus is insonated and made to collapse, thereby liberating mechanical energy on the thrombus surface. This can help fragment the thrombus. Additional research is being performed using thrombus-targeting UCAs in patients receiving a thrombolytic agent so that insonation with the US beam further enhances thrombolysis (referred to as “sonothrombolysis”).11,12
Other therapeutic UCAs are being developed for IV drug delivery to treat a variety of abnormalities, including coronary neointimal hyperplasia and malignancies.13 The use of therapeutic UCAs for diseases such as blood clots and certain ischemic strokes shows promise.14
UCA Administration
Typically, contrast is administered in small (<3 mL) IV bolus injections via a peripheral vein. This provides several minutes of enhancement. If additional contrast enhancement is required, a second administration can be performed. UCAs can also be administered via slow IV infusion to provide prolonged enhancement.15 The additional enhancement time provided by the infusion of contrast is useful for difficult or time-consuming examinations. Infusion of Definity (Lantheus Medical Imaging, N. Billerica, MA) has been approved by the FDA for echocardiographic applications.16
Contrast-Specific Ultrasound Technologies
Although conventional gray-scale US and Doppler flow imaging can be used for CEUS, the results are not ideal and artifacts can be encountered.17 Investigations have been focused on ways to exploit the interactions between US energy and contrast microbubbles in order to improve the clinical utility of CEUS. These investigations have resulted in the development of “contrast-specific” imaging modes such as harmonic imaging, intermittent imaging, and flash-echo imaging. Because the clinical utility of UCAs is greatly improved by the use of these contrast-specific modes, their use is now required during UCA clinical trials and highly preferred when performing clinical CEUS examinations.18
Harmonic Imaging
When subjected to the acoustic energy present in the US imaging field, UCA microbubbles oscillate in size (i.e., they get larger and smaller). The reflected echoes from the oscillating microbubbles contain energy components at the fundamental frequency (i.e., the transmitted frequency) and at higher and lower harmonics (subharmonics).19 In harmonic imaging (HI) mode, the US system is configured to receive only echoes at a particular harmonic frequency of the transmit frequency (e.g., 7.0 MHz for a 3.5 MHz transducer).20,21 When UCAs are imaged with HI mode, the received harmonic echoes from the oscillating microbubbles have a higher signal-to-noise ratio than would be provided by using fundamental US so that regions with microbubbles are more easily appreciated on the resulting gray-scale CEUS image.
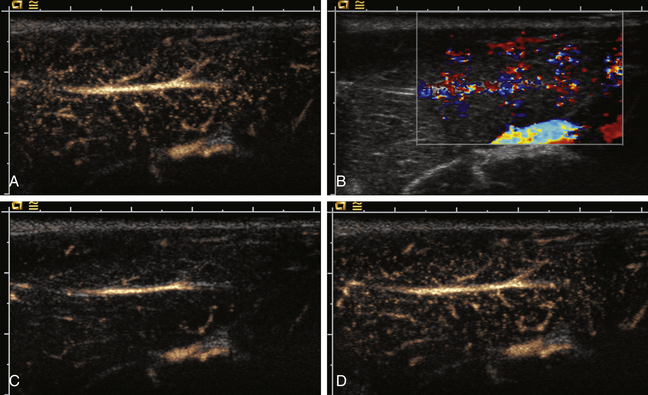
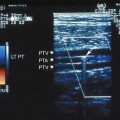
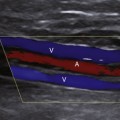
Wide-band HI is a recent advance in HI-mode technology (also referred to as phase-inversion HI and pulse-inversion HI) that employs processing algorithms designed to preferentially display echoes arising from contrast microbubbles and suppress echoes arising from body tissues.22 This imaging technique uses a sequence of two ultrasound pulses that are identical in frequency and amplitude but the second pulse is 180 degrees out of phase with the first pulse. When the two pulses encounter a linear reflector (e.g., body tissue), the resultant echoes cancel one another out, but when the two pulses strike a nonlinear reflector (e.g., contrast microbubble), the harmonic components of the signals combine to result in a signal of higher intensity. Thus, wide-band HI provides a way to better differentiate areas with and without contrast microbubble and has the potential to display blood flow in real-time using gray-scale US, thus obviating the need to use Doppler modes (Figure 4-1). Some scanners can also perform contrast-specific three-dimensional (3D) imaging. Harmonic Doppler US modes (e.g., harmonic power Doppler imaging [PDI], power modulation imaging) have also been developed.22
Low Mechanical Index Imaging and Intermittent Imaging
The energy present within the ultrasound beam can cause microbubble destruction during CEUS examinations.23 Microbubble destruction decreases contrast enhancement by a UCA and therefore decreases the possible clinical utility of the UCA. This must be taken into consideration when using UCAs. One relatively easy way of avoiding this problem is to lower the acoustic output power (i.e., decrease the mechanical index [MI]).
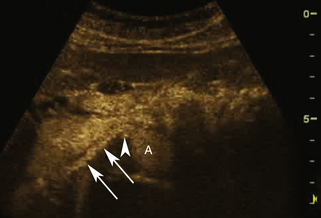
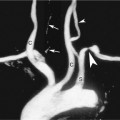
In some cases the intentional destruction of contrast microbubbles in the imaging field is used as a diagnostic tool. For example, CEUS is first used to confirm the presence of contrast material in the target tissue followed by the application of higher acoustic output power (e.g., color Doppler imaging [CDI]) to destroy the microbubbles. After the microbubbles are destroyed, the return of contrast material into the tissue is observed using low-MI CEUS imaging (Figure 4-2). This method can be used to evaluate the rate of re-fill of blood flow (i.e., “reperfusion”) in normal tissues such as the myocardium or skeletal muscles, or to assess neovascularity for tumor characterization.24–26
Intermittent imaging is another method to reduce microbubble destruction.22 In this mode, the system is gated to transmit and receive data at predetermined time intervals (e.g., one pulse every second) or is triggered on a specific portion of the electrocardiogram (e.g., the r wave). Intermittent imaging allows additional microbubbles to enter the field so there is an even greater increase in reflectivity of the contrast-containing vessel or tissue than is possible by continuous real-time imaging. An obvious disadvantage to intermittent imaging is the lack of real-time data. However, there are US systems that provide a dual-image display with high-MI intermittent imaging on one display and low-MI real-time data shown on the other. Several reports have described the clinical potential of using the combination of UCAs and contrast-specific intermittent imaging modes.27–29
Quantification of Contrast Enhancement
The use of UCAs provides quantification capabilities that cannot be obtained using conventional US, such as the ability to assess contrast dynamics (e.g., time to peak enhancement and duration of enhancement), measure changes in signal intensity over time (i.e., time-intensity curves), and compare the transit time (i.e., wash-in, wash-out) of contrast-containing blood through organs and tumors.30–32 Ultrasound systems are available that have on-board calculation packages that can be used to quantify the unique data obtained when UCAs are administered.
Artifacts
The use of UCAs can result in unique imaging artifacts. When imaged with conventional color flow imaging modes, UCAs can cause excessive signal enhancement that causes the display of color Doppler signals beyond the vessel walls.17 This “color blooming” artifact is easily recognized and can usually be eliminated by reducing the color gain setting, increasing the pulse-repetition frequency, or otherwise reducing color sensitivity.
UCAs can also cause artifacts on spectral Doppler displays. For example, contrast-enhanced spectral Doppler waveforms may demonstrate spectral broadening that was not present before contrast administration, whereas microbubble destruction can cause high-intensity spikes on the spectral display. Several reports have indicated that contrast enhancement can increase the peak velocity displayed on Doppler spectral waveforms.33–35 Although this artifact does not affect pulsatility indices or velocity ratios, it should be considered when spectral Doppler is used during contrast-enhanced US examinations. It is also important to recognize that noncontrast Doppler US velocity criteria commonly used to indicate disease may not be appropriate for CEUS examinations.
Clinical Applications of UCAs
The use of CEUS has been investigated for virtually all clinical applications of diagnostic sonography.36–41
The clinical utility of UCAs for echocardiographic examinations is well established, and UCAs are routinely utilized to improve endocardial border definition (EBD) and to assess regional wall motion. Contrast-enhanced echocardiography is also used to improve the detection of intracardiac thrombus, to assess anatomic abnormalities, and for stress echocardiography.42–44
Vascular applications of CEUS include evaluation of the cerebrovascular system, peripheral vessels, and abdominal and retroperitoneal vasculature.45–47 Although qualitative assessments can be performed with conventional color flow imaging modes after contrast administration, more commonly, contrast-specific imaging modes are employed.
Cerebrovascular Applications
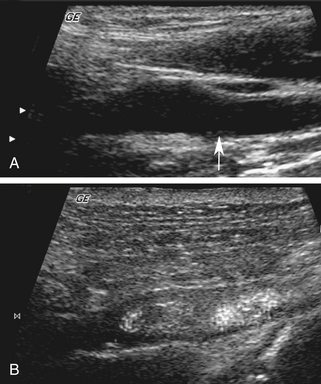
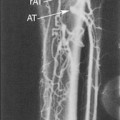
The use of UCAs for the evaluation of the cerebrovascular system includes the intracranial and extracranial blood vessels. The use of CEUS for evaluations of the carotid arteries and other relatively large vessels has the potential to permit direct assessments of the functional lumen and plaque morphology in a manner similar to other imaging modalities such as arteriography and computed tomographic angiography (Figure 4-3).