8 Vascular Disorders of the Spinal Canal
Contents
Vascular Anatomy of the Spinal Cord
Spinal Subarachnoid Hemorrhage
Cavernous Hemangiomas (Cavernomas)
Capillary Telangiectasia and Venous Malformations
Arterial Spinal Cord Infarction
Vascular Anatomy of the Spinal Cord
Knowledge of the normal vascular anatomy of the spinal cord is essential for understanding vascular disorders of the spinal canal.
Arterial Blood Supply
Arterial blood supply systems of the spinal cord
 Longitudinal system
Longitudinal system
 Horizontal system
Horizontal system
The longitudinal system comprises the singular anterior spinal artery and the paired posterior spinal arteries. The spinal arteries arise from the vertebral arteries and the posterior inferior cerebellar arteries at the craniocervical junction. The anterior spinal artery is reinforced along the spinal canal by the anterior radicular arteries, and the posterior spinal arteries are reinforced by the posterior radicular arteries (horizontal system). On average there are 6 anterior and 11–16 posterior radicular arteries that are formed by the nervomedullary arteries. The nervomedullary arteries are supplied from arterial branches coming off the vertebral arteries and also, in the cervical region, from arterial branches coming off the deep cervical, the ascending cervical and the deep intercostal arteries, in the thoracolumbar region from branches derived from the intercostal and lumbar arteries, and in the sacral region from branches of the internal iliac artery. These radicular arteries are less prominent in the mid-thoracic cord. The anterior radicular artery in the thoracolumbar region is the artery with the largest caliber and is known as the great radicular artery or artery of Adamkiewicz (Adamkiewicz, 1882; Lazorthes et al., 1973).
The anterior spinal artery courses in the anterior median fissure from the craniocervical junction to the filum terminale. Together with the anterior radicular arteries, the anterior spinal artery forms the anterior pial arterial plexus and, via segmental penetrating branches (sulcal arteries), supplies about 70 % of the spinal cord, including the corticospinal tracts and the gray matter but excluding the posterior horns (Friedman and Flanders, 1992). The sulcal arteries are arranged in a centrifugal fashion and demonstrate no anastomoses with one another within the spinal cord (terminal arteries; Friedman and Flanders, 1992).
Together with the posterior radicular arteries, the paired posterior spinal arteries form a pial arterial plexus around the dorsal part of the spinal cord. This pial arterial plexus is divided by the denticulate ligament into a dorsal and lateral (vasa corona) pial plexus. Via deep penetrating branches from the dorsal and lateral pial plexus, the posterior spinal arteries supply about 30 % of the spinal cord, including the posterior horns and the dorsal columns (Fig. 8.2) (Friedman and Flanders, 1992). Here the penetrating branches are arranged in a centripetal fashion and demonstrate numerous anastomoses with each other.
In the region of the conus medullaris the anterior spinal artery anastomoses with the paired posterior spinal arteries to form an arterial “basket” (Lazorthes et al., 1958, 1966, 1973; Manelfe, 1973; Lasjaunas and Berenstein, 1990). The blood supply to the filum terminale proceeds around the conus via this “basket” (Djindjian et al., 1988).
The so-called watershed zone of the spinal cord is situated along the gray mater, between the centrifugal (anterior spinal artery) and the centripetal (posterior spinal arteries) penetrating branches (Friedman and Flanders, 1992).
Spinal Cord Veins
Drainage of the spinal cord into the epidural venous plexus is via an intrinsic and an extrinsic venous system.
Intrinsic venous system. Sulcal and horizontal veins are formed from a network of venous capillaries, which exhibit numerous interconnecting horizontal anastomoses and together form sulcal and horizontal veins. Together with transmedullary veins, they form a longitudinal venous plexus that follows the tracts of the gray and white matter.
Extrinsic venous system. The extrinsic system consists of the pial venous plexus, the longitudinal veins (anterior and posterior spinal veins) and the radicular veins. The pial venous plexus, which forms a network of numerous anastomoses on the surface of the spinal cord, connects the intrinsic perforating veins to a system of longitudinal venous anastomoses on the surface of the spinal cord (anterior and posterior spinal veins). The anterior and posterior spinal veins are interconnected by numerous anastomoses and have contact with the radicular veins. Usually (in 60 % of cases) the radicular veins penetrate the dura together with the nerve roots. The radicular veins can also penetrate the dura separately (Lasjaunias and Berenstein, 1990). Although the radicular veins have no valves, they usually display a narrowing at the point of penetration through the dura that assumes the function of an antireflux valve (Tadie et al., 1979). Blood reaches the epidural venous plexus via the anterior and posterior radicular veins.
Epidural venous plexus. There is an extensive network of intercommunicating veins extending from the skull base to the sacrum, which forms the epidural venous plexus. The radicular veins flow either directly into the epidural venous plexus in the region of the neuroforamina or into the anterior epidural venous plexus via a dural pool. The anterior epidural venous plexus is primarily located in the upper cervical and lumbar segments (Rodesch et al., 1992; Gelber et al., 1992). The epidural venous plexus drains into the basivertebral venous plexus located in the vertebral bodies via the radicular veins in the region of the exit points of the foramina intervertebralia.
Spinal Hemorrhage
Classification of spinal hemorrhage according to location
 Spinal epidural hematoma.
Spinal epidural hematoma.
 Spinal subdural hematoma.
Spinal subdural hematoma.
 Spinal subarachnoid hemorrhage.
Spinal subarachnoid hemorrhage.
 Hematomyelia.
Hematomyelia.
Spinal Epidural Hematoma
Etiology and Pathogenesis
Spinal epidural hematomas arise from hemorrhages of the epidural venous plexus. The hemorrhage is situated between the periosteum of the vertebral bodies and the dura mater. The hemorrhage usually extends over several segmental levels and has a predominantly dorsal location. The hemorrhage rapidly leads to compression of the dural sac and subsequently to symptoms of a transverse spinal lesion. It is an urgent indication for MRI (Cowie, 1992; Johnston, 1993).
The most common causes of a spinal epidural hematoma (Gingrich, 1965; Langmayr et al., 1995) are:
• treatment with anticoagulants,
• trauma.
Hemorrhages from vascular malformations are rare.
Incidence, Age and Sex
Spinal epidural hematomas are rare. The age peak lies in the fifth decade of life (Langmayr et al., 1995). Men are more commonly affected than women (Gingrich, 1965).
Clinical Presentation
Spinal epidural hematoma begins with intense, often radiating radicular pain at the level of the hemorrhage. Symptoms of a transverse spinal lesion appear within a few hours.
Diagnostics
MRI
An intraspinal, extramedullary, unenhancing space-occupying lesion appears on MRI, displaying a signal pattern which varies according to the age of the hematoma (Fig. 8.1). GRE sequences are most sensitive for detecting acute hemorrhages, being particularly sensitive to the susceptibility artifacts caused by deoxyhemoglobin. The hematoma itself usually demonstrates a biconvex configuration on sagittal and axial images (Bernsen et al., 1988; Avrahami et al., 1989; Di Lorenzo et al., 1990) and, when located in the anterior part of the epidural space, shows the typical curtain sign, which, because of the Trolard membrane, does not transgress the midline (Fig. 8.2). Hematomas located in the posterior epidural space are not distinguishable by MRI from a subdural hematoma (Fig. 8.3). The surrounding meningeal structures exhibit increased contrast enhancement and may render differentiation from an abscess difficult, depending on the age of the hemorrhage.
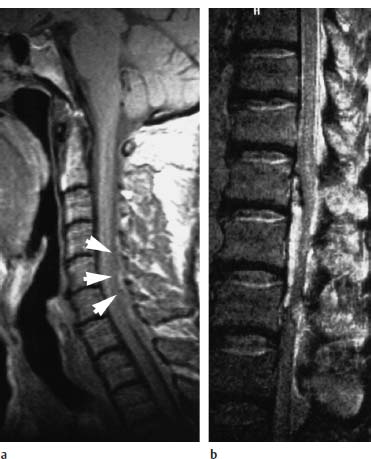
Fig. 8.1a, b Acute epidural hematoma:
a Acute epidural hematomas can be isointense with the spinal cord on the T1-weighted image (arrowheads).
b This patient was in a peracute stage. In this stage the hematoma can have such high water content that it appears predominantly hyperintense on the T2-weighted image, even at 1.5 tesla.
Spinal Subdural Hematoma
Etiology and Clinical Presentation
The etiology of spinal subdural hemorrhage and its clinical presentation are similar to those of an epidural hematoma (Edelson, 1976; Russel and Benoit, 1983). Subdural hematomas are also an urgent indication for MRI (Mattle et al., 1987). The hemorrhage is situated between the layers of the dura mater. Since major blood vessels are lacking below the foramen magnum, a spinal subdural hematoma is very rare. It mainly affects women (Cowie, 1992).
Diagnostics
MRI
Like the epidural hematoma, an unenhancing, intraspinal, extramedullary, space-occupying lesion appears on MRI (Fig. 8.4). Subdural hematomas have a concave configuration on the sagittal image and demonstrate an irregular shape on the axial image (Langmayr et al., 1995).
MRI can only distinguish a subdural hematoma from an epidural hematoma when it is situated in the anterior subdural space and crosses the midline. As already pointed out, the surrounding meninges exhibit increased contrast enhancement and can render differentiation from an abscess difficult, depending on the age of the hemorrhage.
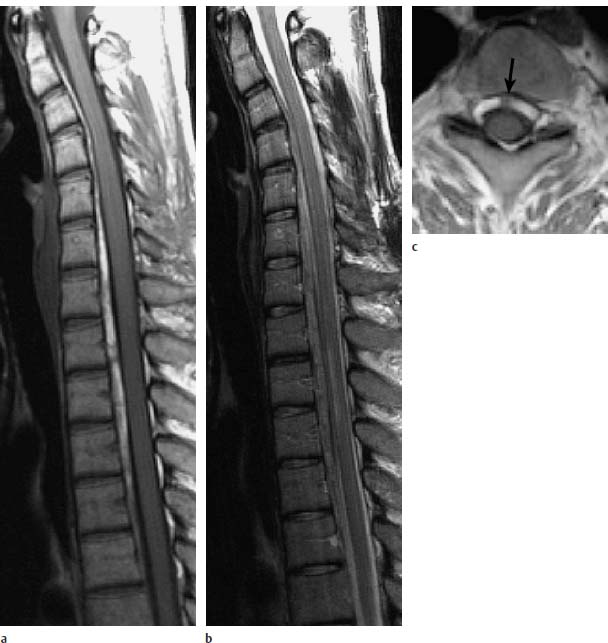
Fig. 8.2a—c Epidural hematoma in a 29-year-old female patient with known systemic lupus erythematosus. Incomplete transverse spinal lesion of 10 days’ duration:
a Sagittal T1-weighted image: Depiction of an intraspinal extramedullar space-occupying lesion extending anteriorly to the spinal cord from C1–T4 and appearing hyperintense. This corresponds to a subacute stage of hemorrhage.
b Sagittal T2-weighted image: The hemorrhage also appears slightly hyperintense on this image. Compression of the spinal cord secondary to edema at the level T1–T3.
c Transverse T1-weighted image: Typical curtain sign indicating that the hematoma is divided into two parts by the Trolard membrane (arrow). This finding is proof of the epidural location.
Spinal Subarachnoid Hemorrhage
Etiology and pathogenesis
The spinal subarachnoid hemorrhage is by far less common than the intracranial subarachnoid hemorrhage. The hemorrhage is located between the spinal cord (pia mater) and the arachnoid membrane.
The most common causes of a spinal subarachnoid hemorrhage are:
• intradural vascular malformations,
• tumors of the spinal cord.
A traumatic spinal subarachnoid hemorrhage is rare. Bleeding from a spinal aneurysm is also a rarity.
Clinical Presentation
Sudden back pain located at the level of the lesion, followed by bilateral sciatica and meningism, is characteristic for a spinal subarachnoid hemorrhage. Intense headache, nausea and vomiting usually ensue within minutes.
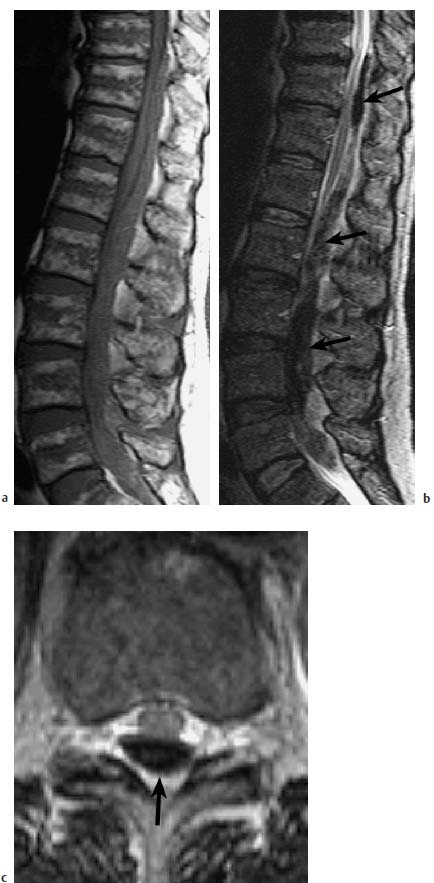
Fig. 8.3a—c Dorsal intraspinal hemorrhage in a 68-year-old patient under anticoagulant therapy. Acute denervation at the L4 level on the left side with bilateral loss of quadriceps reflex and neurogenic bladder:
a Sagittal T1-weighted image: Presentation of an intraspinal space-occupying lesion, which appears isointense, corresponding to a hemorrhage in the acute stage.
b T2-weighted image: The hemorrhage appears hypointense and is associated with a massive intraspinal extramedullary space-occupying effect (arrow).
c Transverse T2-weighted image: Here the hemorrhage appears dorsal in the spinal canal, which makes any reliable differentiation between epi- and subdural location impossible.
Diagnostics
MRI
Like the acute intracranial subarachnoid hemorrhage, the acute spinal subarachnoid hemorrhage is difficult to diagnose by MRI. Distribution and, consequently, dilution of the blood in the CSF renders difficult the detection of susceptibility-type changes caused by deoxyhemoglobin and lead to loss of signal on the T2-weighted images. Detection is easier in the subacute phase due to the paramagnetic effect. The subarachnoid space appears hyperintense on the T1-weighted images. The picture of a spinal CNS siderosis appears during the chronic phase (Fig. 8.5).
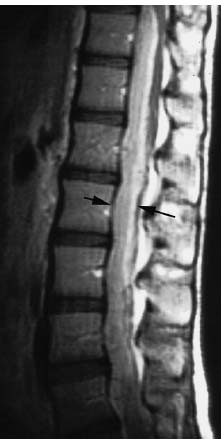
Fig. 8.4 Rare case of a spinal subdural hematoma (arrows) without previous trauma. Signal-intense presentation on the T1-weighted image due to methemoglobin in the subacute stage. Conus and cauda have been displaced dorsally. Sagittal T1-weighted image.
Hematomyelia
Hemorrhage into the spinal cord produces a severe clinical picture and usually extends over several segments.
Etiology and Pathogenesis
Possible causes of hematomyelia are:
 treatment with anticoagulants,
treatment with anticoagulants,
 trauma.
trauma.
More commonly a tumor of the spinal cord, or a vascular malformation, is the root cause of an intramedullary hemorrhage.
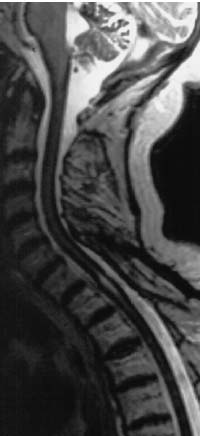
Fig. 8.5 Idiopathic siderosis of the CNS. Sagittal T2-weighted MRI of a 77-year-old female patient. The cervical cord displays the typical superficial reduction in signal due to the susceptibility artifact of the incorporated iron degradation products.
Clinical Presentation
The neurological symptoms with an acute incomplete cord transection syndrome, often in the form of dissociated sensation, are clinically difficult to distinguish from an anterior spinal artery syndrome. Hematomyelia and spinal cord infarction are often clinically inseparable and in these cases MRI is of decisive importance.
Diagnostics
MRI
MRI reveals an intramedullary space-occupying lesion. The signal pattern is related to the age of the hemorrhage. A fresh hemorrhage appears hypointense on T2-weighted images (Fig. 8.6), being displayed most sensitively on GRE sequences due to the higher susceptibility for deoxyhemoglobin. During the acute stage, the hemorrhage appears hyperintense on all weightings due to the paramagnetic effect of methemoglobin. In the chronic stage, hemosiderin causes signal loss, showing up primarily on the T2-weighted images.
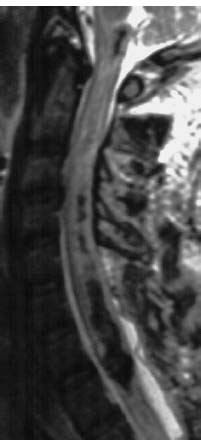
Fig. 8.6 Acute intramedullary hematoma after a traffic accident. A laminectomy to provide relief of symptoms has been performed at the cervicothoracic junction. The acute hemorrhage appears hypointense on the T2-weighted image due to deoxyhemoglobin.
Aneurysms
Spinal aneurysms are rare and usually appear in association with spinal arteriovenous malformations.
Etiology and Pathogenesis
Spinal aneurysms are circumscribed, saccular dilations of the spinal cord arteries or the feeding branches of the spinal cord arteries. Spinal aneurysms are usually associated with arteriovenous malformations (Biondi et al., 1992).
Incidence, Age and Sex
Isolated spinal aneurysms are rare (Rengachary et al. 1993). The incidence is 20 % in patients with intramedullary arteriovenous malformations. The age peak lies between 10 and 40 years. There is no sex predilection (Biondi et al., 1992).
Location
Spinal aneurysms with intramedullary arteriovenous malformations are located on the major feeding branches, which demonstrate, increased blood flow. The majority (70 %) is located on the anterior spinal artery (Biondi et al., 1992). Unlike intracranial aneurysms, spinal aneurysms rarely appear at bifurcations (Rengachary et al., 1993).
Clinical Presentation
In 85 % of cases spinal aneurysms become symptomatic secondary to a subarachnoid hemorrhage. In 15 % of cases they manifest themselves with symptoms of a progressive neurological deficit. Untreated spinal aneurysms usually lead to recurrent hemorrhage (Biondi et al., 1992).
Diagnostics
MRI
To date it has not been possible to demonstrate spinal aneurysms by MRI. MRI is primarily intended to detect a subarachnoid hemorrhage or an intramedullary arteriovenous malformation or to exclude another cause for spinal symptoms. MRI therefore aids in reaching an indication for spinal catheter angiography. The diagnostic reliability of this new method of contrast-enhanced three-dimensional MR angiography is promising, but still requires evaluation.
Spinal Vascular Malformations
Vascular malformations of the spine and spinal cord are rare.
Classification of vascular malformations
 Vascular malformations of the spinal canal:
Vascular malformations of the spinal canal:
– intramedullary arteriovenous malformations,
– intramedullary cavernous hemangiomas (cavernomas),
– perimedullary arteriovenous fistulae,
– dural arteriovenous fistulae with medullary venous drainage.
 Vertebral body hemangiomas.
Vertebral body hemangiomas.
 Paravertebral arteriovenous malformations (located paraspinally or in the intervertebral foramen).
Paravertebral arteriovenous malformations (located paraspinally or in the intervertebral foramen).
Arteriovenous Malformations
Etiology and Pathogenesis
Arteriovenous malformations are classified according to their location, their feeding arteries and their nidus (Manelfe, 1988; Anson and Spetzler, 1992):
Direct arteriovenous fistula. A direct arteriovenous fistula is an intradural, extramedullary arteriovenous shunt. The vascular malformation is situated outside the spinal cord and the pia mater. The arterial inflow is provided by the anterior spinal artery, draining directly into a dilated, elongated venous plexus of varying size. The fistula site demonstrates a high flow rate. The majority of direct arteriovenous fistulae are located anterior to the spinal cord at the level of the conus medullaris.
Arteriovenous malformations with nidus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


