Vascular Lesions
Aneurysms
The incidence in the normal population of saccular (berry) aneurysms differs widely between reports, but is likely well below 5%. They are multiple in 15% of cases. Patients with adult polycystic kidney disease and Marfan syndrome are at greater risk for an intracranial aneurysm. The clinical presentation is that of subarachnoid hemorrhage. Saccular aneurysms occur at arterial branching points, with 90% in the anterior circulation and 10% in the posterior circulation. Thirty percent involve the origin of the anterior communicating artery, 30% the origin of the posterior communicating artery, and 20% the middle cerebral artery trifurcation ( Fig. 1.78 ). The most common location in the posterior circulation is the basilar tip, followed by the origin of the PICA.

Although a small saccular aneurysm may be visualized on a conventional MR or CT scan, three-dimensional (3D) TOF MRA and CTA are specifically employed for detection (and delineation). CTA is the modality of choice in the acute presentation with subarachnoid hemorrhage, whereas 3D TOF MRA is often used for detection and evaluation of asymptomatic aneurysms, as well as for correlation and further definition of lesions in the acute setting and on follow-up. Modern scanners easily detect aneurysms as small as 2 mm in diameter. Treatment of intracranial brain aneurysms that have bled, or are deemed to present a significant risk to the patient because of potential bleeding in the future, involves either surgical clipping or endovascular occlusion. Surgery is much less common today, although not all aneurysms can currently be treated endovascularly.
In the acute setting, with a small, ruptured aneurysm, it is well known that a small percentage of patients can have a normal x-ray angiogram (DSA). This is presumed to be due to vasospasm and mass effect, with nonfilling of the aneurysm, despite the abundant subarachnoid blood. It stands to reason that MRA and CTA will also be negative in this instance.
A giant intracranial aneurysm is by definition a saccular aneurysm with a diameter > 25 mm. These make up a small percentage of all intracranial aneurysms. Clinical presentation may be due to mass effect (cranial nerve palsies) or rupture (subarachnoid hemorrhage). Giant aneurysms most commonly involve the cavernous or supraclinoid internal carotid artery and basilar terminus.
In regards to MR technique, there are a few caveats. Giant aneurysms may not be depicted in their entirety on 3D TOF MRA due to slow flow within the aneurysm, and in this instance correlation with two-dimensional (2D) TOF and conventional images, or additional contrast-enhanced 3D MRA, can be helpful in delineating the true aneurysm extent. Large aneurysms may also be associated with pulsation artifacts on MR, which can help to confirm the vascular nature of the lesion on conventional scans. On a flow study such as CTA or MRA, the size of even a small saccular aneurysm can be underestimated due to the presence of thrombus. Comparison of conventional MR scans with source images from the MRA exam improves recognition of thrombus. On rare occasion, with giant aneurysms, layered thrombus is visible on conventional images.
Mycotic aneurysms are caused by septic emboli. Their location is usually peripheral, in distinction to saccular aneurysms. Most are bacterial, with a fungal origin less common. Treatment is generally with antibiotics. Serial evaluation (by MR, CTA, or DSA) is recommended to assess for possible enlargement. The mortality rate is high with rupture. Conventional CT and MR imaging is nonspecific in regard to appearance, demonstrating a small enhancing lesion with surrounding cerebral edema.
Vascular Malformations
An arteriovenous malformation (AVM) consists of a nidus (tangle) of tightly packed, dilated, tortuous arteries and veins, without an intervening capillary network, with the result being arteriovenous shunting. It is the most common symptomatic vascular malformation of brain. The risk of hemorrhage is 2 to 3% per year, with each episode having a 30% risk of death. Most lesions present clinically between 20 and 40 years of age, and involve peripheral branches of the ACA or MCA. Aneurysms of the feeding arteries (perinidal aneurysms), due to high flow, are seen in less than 10% of cases.
AVMs are well depicted on conventional MR imaging (due to flow phenomena), with TOF MRA used to better demonstrate the nidus, enlarged arterial feeding vessels, and enlarged draining veins ( Fig. 1.79 ). On occasion, a small AVM will be visualized only on MRA. On precontrast conventional MR scans, multiple serpiginous vessels, most with low signal intensity (SI) due to rapid flow, are seen. Contrast enhancement often provides improved visualization of the nidus, together with the enlarged draining veins. Between the large draining veins there will be preserved normal brain parenchyma. Gliosis is uncommon. There is usually little mass effect, with vasogenic edema unusual. On unenhanced CT, even large lesions may be missed. Calcification is seen in the minority of cases. Enhancement on CT provides visualization of the nidus and large draining veins.

The vein of Galen aneurysmal malformation is easily recognized on MRI or enhanced CT due to the presence of a large midline varix in the neonate or infant ( Fig. 1.80 ). The epicenter of the varix is in the quadrigeminal plate cistern. Presenting symptoms are due to high-output cardiac failure, with embolization the treatment of choice.

A dural arteriovenous fistula (AVF) is an acquired vascular malformation of the brain that is most commonly seen in the posterior fossa. The etiology is believed to be occlusion of a venous sinus, with recanalization along the walls of the sinus leading to numerous direct connections between small feeding arteries and venous drainage. Clinical complications include venous infarction, parenchymal hemorrhage, and subdural hematoma. On MR and enhanced CT large superficial dural-based veins may be identified, without a parenchymal nidus ( Fig. 1.81 ).

There are two types of carotid-cavernous fistulas (CCFs): direct and indirect. Most direct CCFs are traumatic in etiology, secondary to skull base fracture. These are high flow lesions. Indirect CCFs are low flow, nontraumatic acquired lesions, representing an AVF of the cavernous sinus with branches either of the external carotid artery or the cavernous carotid artery. With direct CCFs, findings include proptosis, dilatation of the superior ophthalmic vein(s), and enlargement of the cavernous sinus (es) ( Fig. 1.82 ). Prominent flow voids will be present in the cavernous sinus on MR. DSA is the definitive exam for diagnosis. On DSA, early filling of the cavernous sinus, together with retrograde filling of the superior ophthalmic vein, can be seen. There may be reduced flow in the internal carotid artery beyond the fistula.

Detection of an indirect CCF on MR and CT can be difficult. Enlargement of the superior ophthalmic vein(s) is perhaps the most consistent and earliest finding. As with other AVFs, post-contrast imaging may demonstrate increased vascularity surrounding one or both cavernous sinuses. Unlike direct CCFs, the cavernous sinuses are typically not as prominent with an indirect CCF. Indirect CCFs are rarely detected by non-DSA imaging before symptoms are observed clinically.
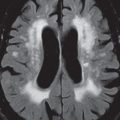
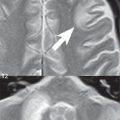
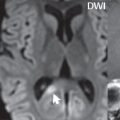
A cerebral cavernous malformation (CCM, previously known as a cavernous angioma) histologically consists of a honeycomb of vascular spaces, separated by fibrous strands, without intervening normal brain parenchyma ( Fig. 1.83 ). Seventy-five percent are supratentorial, with multiplicity common (25%) ( Fig. 1.84 ). They are prone to spontaneous hemorrhage and, for symptomatic lesions in at risk patients, are treated by surgical resection ( Fig. 1.85 ).



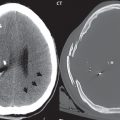
As with almost all brain disease (in particular parenchymal lesions), MR is the modality of choice for detection, differential diagnosis, and evaluation. A CCM will have mixed low and high SI on both T1- and T2-weighted images (a “popcorn ball” appearance), with a complete hemosiderin rim. The latter is best visualized on gradient echo (GRE) T2-weighted images, as low signal intensity. Susceptibility weighted imaging (SWI) provides a further improvement in visualization of the hemosiderin rim, as well as improved detection of very small lesions. Mild heterogeneous contrast enhancement is common with all but the smallest lesions. On CT, large CCMs can be visualized, and present as focal high-density lesions, with associated calcification. They may be associated with a developmental venous anomaly (DVA).
The more descriptive term developmental venous anomaly (DVA) is now used in place of the older term venous angioma. In this entity, a stellate collection of peripheral veins (the “caput medusae”) lying in white matter converge to a dilated central draining vein, an appearance well visualized on contrast-enhanced MR. Supratentorial DVAs typically drain toward the wall of the lateral ventricle whereas infratentorial lesions may drain into the sigmoid sinus ( Fig. 1.86 ). DVAs are usually solitary, are commonly visualized incidentally on contrast-enhanced MR, and are the most common asymptomatic vascular malformation of brain.

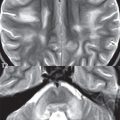
A capillary telangiectasia is histologically a cluster of enlarged, dilated capillaries interspersed with normal brain parenchyma. These are rare, clinically benign, lesions with the most common site being the pons (often centrally) ( Fig. 1.86 ). MR imaging characteristics include a size < 1 cm, faint contrast enhancement, hypointensity on GRE and SWI, and in about half of cases faint hyperintensity on FLAIR. These are usually visualized incidentally, typically being quiescent without symptoms.
Vertebrobasilar dolichoectasia by definition involves both an increase in length (“dolicho”) and diameter (“ectasia”) of the involved vessels ( Fig. 1.87 ). This entity is seen in the elderly with hypertension and atherosclerotic disease. Regarding the basilar artery, elongation results in the artery lying lateral to the clivus or dorsum sellae, or terminating above the suprasellar cistern. A diameter > 4.5 mm on CT is considered to be ectatic. Cranial nerve deficits may occur. There can be marked (long standing) deformity of the pons. However, vertebrobasilar dolichoectasia by itself is typically asymptomatic.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree