Vascular malformations arise from disorganized angiogenesis and manifest as congenital abnormalities that may be isolated events or associated with syndromes. The International Society for the Study of Vascular Anomalies (ISSVA) recognizes vascular malformations as a separate entity from vascular tumors and classifies vascular malformations into four major groups: simple, combined, anomalies of major vessels, and those associated with other vascular anomalies. Vascular tumors, including infantile hemangioma, rapidly involuting congenital hemangioma, noninvoluting congenital hemangioma, and kaposiform hemangioendothelioma are discussed in Chapter 16 .
Imaging Vascular Malformations
Vascular malformations may be imaged using several modalities. Ultrasound, often the first line imaging modality for a suspected vascular malformation, is helpful for the identification of superficial and deep malformations without using ionizing radiation. The added utility of color and spectral Doppler can accurately define venous and arterial anatomy, and provide nidus size and the number of feeding and draining vessels. It can also be used to assess the presence or absence of a deep venous system in affected extremities. MRI, however, is the mainstay in the imaging of most vascular malformations, owing to its superior contrast resolution and techniques providing temporal resolution. A typical protocol for imaging malformations uses conventional nonenhanced T1-weighted fast spin echo (T1W FSE), contrast-enhanced T1W FSE with fat suppression, and T2W FSE with fat suppression. Time-resolved angiographic sequences provide temporal resolution and can accurately identify abnormal arterial and venous structures and help to assess lesion enhance patterns.
Table 15.1 provides a summary of the imaging findings expected with the vascular malformations discussed in subsequent sections.
| Ultrasound | Magnetic Resonance Imaging | ||||
|---|---|---|---|---|---|
| Malformation | Grayscale | Doppler | T2W | T1W | T1W+C |
| Slow Flow | |||||
| Capillary |
|
|
|
|
|
| Lymphatic | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| Venous |
|
|
|
|
|
| Fast Flow | |||||
| Arteriovenous |
|
|
|
|
|
| Arteriovenous fistula |
|
|
|
|
|
Simple Vascular Malformations
Simple vascular malformations are subdivided into low- and high-flow malformations. Low-flow malformations include capillary, venous, and lymphatic malformations, whereas high-flow malformations include arteriovenous malformations (AVMs) and fistulas.
Capillary Malformations
Capillary malformations (CM) are the most common vascular malformation and histologically present as disorganized capillary vessels embedded in the skin and subcutaneous tissues. Because of their superficial nature, CM presents as a cutaneous discoloration that is often pink-red and eponymously referred to as a port-wine stain. These lesions are frequently diagnosed on direct inspection, prompting further evaluation. CMs are infrequently imaged in isolation, with imaging often performed to identify larger vascular malformations deeper within the body. Imaging manifestations of CM may show skin or subcutaneous tissue thickening that may enhance after intravenous contrast administration. Sonographic findings of a port-wine stain are nonspecific and can demonstrate skin thickening with or without flow on color Doppler imaging.
Lymphatic Malformations
Lymphatic malformations (LM) arise from disorganized formation of endothelialized lymphatic channels that neither contribute nor connect to normal lymphatic channels. Histologically, terms such as cystic hygroma or lymphangioma are inaccurate and not favored, as the terms give a false suggestion of a vascular (“-angio-”) or neoplastic (“-oma”) component. LMs are subdivided into either microcystic or macrocystic groups, and some lesions may be a combination of the two entities with both microcystic and macrocystic components.
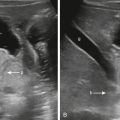
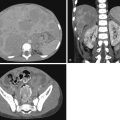
Macrocystic LMs present as fluid-filled structures greater than 1 to 2 cm and will appear hyperintense on T2-weighted imaging. Thin septations are often seen separating larger cystic spaces, and fluid–fluid levels are sometimes seen as a result of hemorrhage into a cyst ( Fig. 15.1 ). Microcystic LMs appear to mimic “solid” masses with lymphatic channels that are often too small to be seen. Septal enhancement on postcontrast-enhanced imaging is variable and may appear as homogeneous or slightly heterogeneous in the setting of microcystic LM. Cystic spaces should not enhance after the administration of intravenous contrast. Sonographic findings of LM correlate to the MRI findings, with large anechoic cystic spaces and septations. Microcystic lesions appear as echogenic soft tissues from the multiple reflectors of the small varying tissue interfaces between microscopic lymphatic channels and surrounding tissue ( Fig. 15.2 ).


The most common region of the body for an LM is the head and neck, but an LM may be seen anywhere. When found in the head and neck, special considerations such as airway evaluation for patency as well as swallowing mechanics must be assessed. LMs are susceptible to superinfection and cellulitis, especially after hemorrhage into a cystic lesion. In inflammatory or hemorrhagic states, LMs increase in size and may cause airway compromise.
First line treatment of macrocystic LMs is sclerotherapy. LMs are responsive to oral sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR)and are especially helpful when treating LMs and venous malformations (VMs) associated with mutations in the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin ( PI3K/AKT/mTOR ) pathway. Side effects of sirolimus include immunosuppression, and medical management of patients on mTOR inhibitors is best managed by hematologists with multidisciplinary care teams. Surgical excision of an LM is reserved for lesions too large to be amenable to therapy.
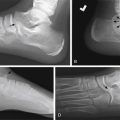
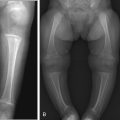
Venous Malformations
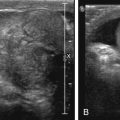
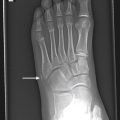
Venous malformations (VM) are low-flow malformations. These lesions may present with concomitant lymphatic channels as a combined lymphatic-venous malformation (LVM). They are dysplastic congenital anomalies that may be focal or multifocal and are sometimes transspatial. VMs may present as dilated venous varicosities ( Fig. 15.3 ) or as a spongiform conglomerate of venous channels that sonographically appear as tubular-like anechoic or hypoechoic foci with either no discernable flow with color and spectral Doppler imaging, or monophasic venous flow present sometimes only after augmentation with Valsalva maneuvers, compression, or crying ( Fig. 15.4 ). VMs are classically hyperintense on T2-weighted MRI sequences due to the slow flow within the aberrant channels; time-resolved contrast-enhanced MRA will demonstrate slow progressive filling of venous channels and delayed postcontrast imaging of a VM will characteristically demonstrate enhancement of venous channels. T1- and T2-hypointense foci within a VM represent phleboliths. Like LMs, fluid–fluid levels can be appreciated in VMs as a result of stagnant flow with hematocrit or protein layering or hemorrhage ( Fig. 15.5 ). VMs may present radiographically with phleboliths.