Chapter SEVEN
Vascular Radiology
CHARLES Y. KIM  CHAPTER EDITOR
CHAPTER EDITOR
GLENN
E. NEWMAN
AND
CHARLES Y. KIM
HISTORY
A 45-year-old woman with diabetes mellitus and exercise claudication. Both femoral artery pulses are markedly diminished and the popliteal and pedal pulses are detectable only by Doppler recording. The ankle-brachial index (ABI) at rest was 0.48 on the right and 0.47 on the left.
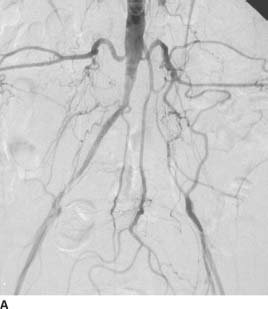
 FIGURE 7-1A Pelvic arteriogram with catheter tip in the distal aorta shows that the left common iliac artery is occluded and is reconstituted by multiple collateral arteries from lumbar arteries, the middle sacral artery, and the IMA. A severe stenosis of the right external iliac artery is also present.
FIGURE 7-1A Pelvic arteriogram with catheter tip in the distal aorta shows that the left common iliac artery is occluded and is reconstituted by multiple collateral arteries from lumbar arteries, the middle sacral artery, and the IMA. A severe stenosis of the right external iliac artery is also present.
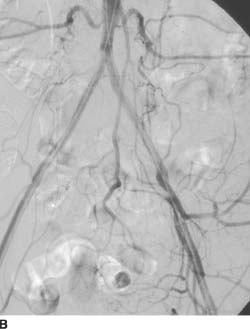
 FIGURE 7-1B Repeat arteriogram after successful an-gioplasty and deployment of so-called kissing common iliac stents, extended to the bilateral external iliac arteries, shows restored patency of both common iliac and external iliac arteries.
FIGURE 7-1B Repeat arteriogram after successful an-gioplasty and deployment of so-called kissing common iliac stents, extended to the bilateral external iliac arteries, shows restored patency of both common iliac and external iliac arteries.
DIFFERENTIAL DIAGNOSIS
 Atherosclerotic peripheral vascular disease (PVD): This diagnosis is by far the most common etiology of arterial occlusion in adults, particularly in those with risk factors such as diabetes mellitus, smoking, and hypertension. Atherosclerosis is a slow, chronic progressive disease, which allows formation of well-developed collateral arteries. Diffuse arterial involvement is typical. Given the patient’s risk factors, extensive collateral circulation, and diffuse involvement, this is the most likely diagnosis.
Atherosclerotic peripheral vascular disease (PVD): This diagnosis is by far the most common etiology of arterial occlusion in adults, particularly in those with risk factors such as diabetes mellitus, smoking, and hypertension. Atherosclerosis is a slow, chronic progressive disease, which allows formation of well-developed collateral arteries. Diffuse arterial involvement is typical. Given the patient’s risk factors, extensive collateral circulation, and diffuse involvement, this is the most likely diagnosis.
 Vasculitis: Vasculitis of large arteries such as Takayasu’s arteritis and giant cell arteritis typically produce smooth stenoses, but occasionally occlusions can occur. These patients tend to be younger than the patient shown in this case, with an elevated erythrocyte sedimentation rate (ESR). Additionally, constitutional symptoms of fever, arthralgias, myalagias, rash, and fatigue, which are absent in this case, are often noted. The luminal irregularity of the aorta and iliac arteries, age, and comorbidities make this diagnosis unlikely.
Vasculitis: Vasculitis of large arteries such as Takayasu’s arteritis and giant cell arteritis typically produce smooth stenoses, but occasionally occlusions can occur. These patients tend to be younger than the patient shown in this case, with an elevated erythrocyte sedimentation rate (ESR). Additionally, constitutional symptoms of fever, arthralgias, myalagias, rash, and fatigue, which are absent in this case, are often noted. The luminal irregularity of the aorta and iliac arteries, age, and comorbidities make this diagnosis unlikely.
 Embolic occlusion: This acute process produces acute symptoms of ischemia, with an embolic source typically originating from the heart. Angiographically, due to the acuity of this process, collateral circulation would be expected to be absent or minimal (unlike the substantial collateral arteries seen in the case presented here). This diagnosis unlikely.
Embolic occlusion: This acute process produces acute symptoms of ischemia, with an embolic source typically originating from the heart. Angiographically, due to the acuity of this process, collateral circulation would be expected to be absent or minimal (unlike the substantial collateral arteries seen in the case presented here). This diagnosis unlikely.
 Arterial dissection: Usually, some degree of flow is preserved, although any dissection can progress to complete occlusion. Dissection of the iliac arteries is usually seen only after recent arterial intervention (which is absent prior to the image shown in Figure 7-1A). Spontaneous dissection of the iliac arteries is very rare. Angiographically, linear margins or an actual dissection flap are typically visualized; these findings are absent in the case shown. Given the chronic appearance, diffuse arterial involvement, and lack of history of recent arterial intervention, this diagnosis is unlikely.
Arterial dissection: Usually, some degree of flow is preserved, although any dissection can progress to complete occlusion. Dissection of the iliac arteries is usually seen only after recent arterial intervention (which is absent prior to the image shown in Figure 7-1A). Spontaneous dissection of the iliac arteries is very rare. Angiographically, linear margins or an actual dissection flap are typically visualized; these findings are absent in the case shown. Given the chronic appearance, diffuse arterial involvement, and lack of history of recent arterial intervention, this diagnosis is unlikely.
DIAGNOSIS
Atherosclerotic peripheral vascular disease
Clinical
 Claudication is the development of cramping pain in the legs during exercise, related to insufficient arterial inflow to accommodate muscular demand. Physical examination findings include diminished or absent peripheral arterial pulses and trophic changes in the affected extremities such as hair loss and muscle atrophy.
Claudication is the development of cramping pain in the legs during exercise, related to insufficient arterial inflow to accommodate muscular demand. Physical examination findings include diminished or absent peripheral arterial pulses and trophic changes in the affected extremities such as hair loss and muscle atrophy.
 Obstructive atherosclerotic disease of the infrarenal segment of the abdominal aorta and pelvic arteries supplying the legs is relatively common, with a prevalence of approximately 40/10,000 patients.
Obstructive atherosclerotic disease of the infrarenal segment of the abdominal aorta and pelvic arteries supplying the legs is relatively common, with a prevalence of approximately 40/10,000 patients.
 Obstructive PVD is more common in men than women. It generally occurs after 50 years of age unless a concomitant disease such as diabetes mellitus or a family history of atherosclerotic disease is present.
Obstructive PVD is more common in men than women. It generally occurs after 50 years of age unless a concomitant disease such as diabetes mellitus or a family history of atherosclerotic disease is present.
 Integration of the history, physical examination, and results of noninvasive tests allows preangiographic prediction of the disease site and severity, including the presence of inflow (i.e., suprainguinal) and outflow (i.e., infrainguinal) disease.
Integration of the history, physical examination, and results of noninvasive tests allows preangiographic prediction of the disease site and severity, including the presence of inflow (i.e., suprainguinal) and outflow (i.e., infrainguinal) disease.
 The ABI, a ratio of the ankle blood pressure relative to the arm blood pressure, is used to predict the presence and severity of aortoiliac and lower extremity atherosclerotic disease. An ABI of ≥0.9 is considered normal; mild insufficiency: 0.7 to 0.9; moderate insufficiency: 0.5 to 0.7; severe insufficiency (i.e., a threatened limb): <0.5.
The ABI, a ratio of the ankle blood pressure relative to the arm blood pressure, is used to predict the presence and severity of aortoiliac and lower extremity atherosclerotic disease. An ABI of ≥0.9 is considered normal; mild insufficiency: 0.7 to 0.9; moderate insufficiency: 0.5 to 0.7; severe insufficiency (i.e., a threatened limb): <0.5.
 The choice of therapies is determined by the anatomic location and extent of arterial disease and comorbid features (e.g., cardiopulmonary status). Approximately 90% of patients have a clinical history of atherosclerotic disease in other locations—for example, coronary or carotid artery circulation.
The choice of therapies is determined by the anatomic location and extent of arterial disease and comorbid features (e.g., cardiopulmonary status). Approximately 90% of patients have a clinical history of atherosclerotic disease in other locations—for example, coronary or carotid artery circulation.
Radiologic
 Noninvasive imaging studies such as computed tomography angiography (CTA) and MR angiography (MRA) are often utilized for diagnosis and treatment-planning purposes.
Noninvasive imaging studies such as computed tomography angiography (CTA) and MR angiography (MRA) are often utilized for diagnosis and treatment-planning purposes.
 CTA and MRA have an equally high sensitivity and specificity for detecting and characterizing stenoses and occlusions of the pelvic and lower extremity arteries. However, these techniques have some limitations. Degree of stenosis may be overestimated in diseased arteries. Although underestimation of degree of stenosis can also occur, it is a less common problem. In addition, prominent vascular calcifications can limit assessment of stenoses with CTA.
CTA and MRA have an equally high sensitivity and specificity for detecting and characterizing stenoses and occlusions of the pelvic and lower extremity arteries. However, these techniques have some limitations. Degree of stenosis may be overestimated in diseased arteries. Although underestimation of degree of stenosis can also occur, it is a less common problem. In addition, prominent vascular calcifications can limit assessment of stenoses with CTA.
 Conventional (catheter) angiography continues to be the reference standard for arterial imaging; findings are not altered by the presence of vascular calcifications.
Conventional (catheter) angiography continues to be the reference standard for arterial imaging; findings are not altered by the presence of vascular calcifications.
 The major aim of radiologic assessment is to preoperatively determine the type and extent of disease, which will guide therapy. Imaging features of importance include the degree of stenosis, presence of occlusion, number of lesions, length of stenosis or occlusion, degree of calcification, luminal irregularity, and extent and location of collateral vessels. The arteries providing flow along the entire course from the aorta to the foot should be evaluated before any therapy is planned.
The major aim of radiologic assessment is to preoperatively determine the type and extent of disease, which will guide therapy. Imaging features of importance include the degree of stenosis, presence of occlusion, number of lesions, length of stenosis or occlusion, degree of calcification, luminal irregularity, and extent and location of collateral vessels. The arteries providing flow along the entire course from the aorta to the foot should be evaluated before any therapy is planned.
 Percutaneous angioplasty (PTA), alone or in combination with intravascular stenting, is the major nonsurgical means of treating atherosclerotic aortoiliac disease. Endovascular intervention is not possible when occlusive lesions cannot be crossed with a guidewire.
Percutaneous angioplasty (PTA), alone or in combination with intravascular stenting, is the major nonsurgical means of treating atherosclerotic aortoiliac disease. Endovascular intervention is not possible when occlusive lesions cannot be crossed with a guidewire.
 Although PTA alone is frequently successful, stenting is sometimes also necessary. Indications for stenting include persistent stenosis (>30%) after PTA and flow-limiting arterial dissection at the site of angioplasty. Either a self-expanding or a balloon-mounted stent can be used.
Although PTA alone is frequently successful, stenting is sometimes also necessary. Indications for stenting include persistent stenosis (>30%) after PTA and flow-limiting arterial dissection at the site of angioplasty. Either a self-expanding or a balloon-mounted stent can be used.
 Factors that predict long-term patency after PTA can be divided into two categories: (1) clinical predictors and (2) anatomic predictors.
Factors that predict long-term patency after PTA can be divided into two categories: (1) clinical predictors and (2) anatomic predictors.
 Clinical predictors are based on the symptoms before PTA. In general, patients who present with claudication fare much better overall than those who present for limb salvage (e.g., nonhealing ulcers, rest pain, or gangrene). This finding is expected because the latter is a reflection of much more extensive vascular disease.
Clinical predictors are based on the symptoms before PTA. In general, patients who present with claudication fare much better overall than those who present for limb salvage (e.g., nonhealing ulcers, rest pain, or gangrene). This finding is expected because the latter is a reflection of much more extensive vascular disease.
 Anatomic predictors are based on the location and angiographic appearance of the lesion. Patients with proximal lesions (i.e., so-called inflow disease) have a much better prognosis than those with more distal lesions. Angiographic lesion parameters include the degree of vessel narrowing, the degree of irregularity of the lesion, and its length. In general, patients with stenoses have a better result from PTA than those with occlusions. In addition, patients with short (i.e., <5 cm), concentric (smooth) lesions fare better than those with long (i.e., >5 cm), eccentric (irregular) lesions.
Anatomic predictors are based on the location and angiographic appearance of the lesion. Patients with proximal lesions (i.e., so-called inflow disease) have a much better prognosis than those with more distal lesions. Angiographic lesion parameters include the degree of vessel narrowing, the degree of irregularity of the lesion, and its length. In general, patients with stenoses have a better result from PTA than those with occlusions. In addition, patients with short (i.e., <5 cm), concentric (smooth) lesions fare better than those with long (i.e., >5 cm), eccentric (irregular) lesions.
 The 5-year patency rate of iliac PTA alone is approximately 70% to 75%, but that of PTA with intravascular stenting is 80% to 85%.
The 5-year patency rate of iliac PTA alone is approximately 70% to 75%, but that of PTA with intravascular stenting is 80% to 85%.
 Simultaneous bilateral iliac angioplasty or stenting (so-called kissing iliac angioplasty and/or stenting) is indicated for stenosis or occlusion at the origin of the common iliac artery, whether unilateral or bilateral. Angioplasty and stenting are performed simultaneously, in order to prevent plaque being pushed into the contralateral common iliac artery origin.
Simultaneous bilateral iliac angioplasty or stenting (so-called kissing iliac angioplasty and/or stenting) is indicated for stenosis or occlusion at the origin of the common iliac artery, whether unilateral or bilateral. Angioplasty and stenting are performed simultaneously, in order to prevent plaque being pushed into the contralateral common iliac artery origin.
 Aortobifemoral bypass graft surgery is preferred for patients who have extensive atherosclerotic disease and low surgical risk.
Aortobifemoral bypass graft surgery is preferred for patients who have extensive atherosclerotic disease and low surgical risk.
SUGGESTED READING
Kashyap VS, Pavkov ML, Bena JF, et al. The management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstruction. J Vasc Surg 2008;48(6):1451–1457, 1457.e1–e3.
Mouanoutoua M, Maddikunta R, Allaqaband S, et al. Endovascular intervention of aortoiliac occlusive disease in high-risk patients using the kissing stents technique: long-term results. Catheter Cardiovasc Interv 2003;60(3):320–326.
Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endo-vasc Surg 2007;33(Suppl 1):S1–S75.
Sharafuddin MJ, Hoballah JJ, Kresowik TF, et al. Long-term outcome following stent reconstruction of the aortic bifurcation and the role of geometric determinants. Ann Vasc Surg 2008;22(3):346–357.
Willmann JK, Wildermuth S, Pfammatter T, et al. Aortoiliac and renal arteries: prospective intraindividual comparison of contrastenhanced three-dimensional MR angiography and multi-detector row CT angiography. Radiology 2003;226(3):798–811.
TONY P.
SMITH
HISTORY
A 40-year-old woman with nephrotic syndrome and sudden onset of right-sided pleuritic chest pain.
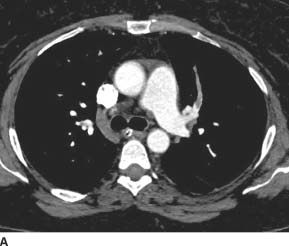
 FIGURE 7-2A Axial contrast-enhanced CT image through the main pulmonary artery shows an occlusive filling defect in the left upper lobe anterior segmental pulmonary artery. This proximal margin of the filling defect has a “meniscus” appearance, which argues strongly that it is a real finding rather than an artifact.
FIGURE 7-2A Axial contrast-enhanced CT image through the main pulmonary artery shows an occlusive filling defect in the left upper lobe anterior segmental pulmonary artery. This proximal margin of the filling defect has a “meniscus” appearance, which argues strongly that it is a real finding rather than an artifact.
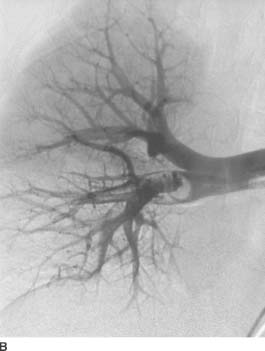
 FIGURE 7-2B Right pulmonary artery angiogram. A filling defect is seen in the interlobar artery extending into the right middle and lower lobe branches.
FIGURE 7-2B Right pulmonary artery angiogram. A filling defect is seen in the interlobar artery extending into the right middle and lower lobe branches.
 Pulmonary thromboembolus: A filling defect within a pulmonary artery, as is seen in the case shown here, is essentially diagnostic for pulmonary embolism (PE). This diagnosis is correct.
Pulmonary thromboembolus: A filling defect within a pulmonary artery, as is seen in the case shown here, is essentially diagnostic for pulmonary embolism (PE). This diagnosis is correct.
 Pulmonary vein thrombosis: Occasionally a filling defect within a pulmonary vessel is due to a pulmonary vein thrombosis. Tracing the vessel to its origin reveals whether it is pulmonary artery or pulmonary vein. In the case shown, the vessel arises from the main pulmonary artery rather than the vein. Pulmonary venous thrombosis is an incorrect diagnosis.
Pulmonary vein thrombosis: Occasionally a filling defect within a pulmonary vessel is due to a pulmonary vein thrombosis. Tracing the vessel to its origin reveals whether it is pulmonary artery or pulmonary vein. In the case shown, the vessel arises from the main pulmonary artery rather than the vein. Pulmonary venous thrombosis is an incorrect diagnosis.
 Air embolus: The density of the filling defect in an air embolus would appear the same as that of the lung. However, in the case shown, the density of the filling defect is similar to that of muscle. Therefore, this diagnosis is incorrect.
Air embolus: The density of the filling defect in an air embolus would appear the same as that of the lung. However, in the case shown, the density of the filling defect is similar to that of muscle. Therefore, this diagnosis is incorrect.
 Artifact: A filling defect could be due to artifact from volume averaging or motion. Inspection of adjacent contiguous images should help determine whether the finding is a true filling defect or artifact. Artifacts tend to have linear margins whereas true filling defects have a so-called meniscus appearance at their margin, which is present in this case. Therefore, artifact is an incorrect diagnosis.
Artifact: A filling defect could be due to artifact from volume averaging or motion. Inspection of adjacent contiguous images should help determine whether the finding is a true filling defect or artifact. Artifacts tend to have linear margins whereas true filling defects have a so-called meniscus appearance at their margin, which is present in this case. Therefore, artifact is an incorrect diagnosis.
DIAGNOSIS
Acute pulmonary thromboembolus
KEY FACTS
Clinical
 PE is a common, life-threatening entity with an estimated 600,000 cases per year in the United States alone. Approximately 10% of the patients with PE do not survive the initial event.
PE is a common, life-threatening entity with an estimated 600,000 cases per year in the United States alone. Approximately 10% of the patients with PE do not survive the initial event.
 The clinical signs of PE are nonspecific and typically can include dyspnea, tachypnea, pleuritic pain, and hemoptysis.
The clinical signs of PE are nonspecific and typically can include dyspnea, tachypnea, pleuritic pain, and hemoptysis.
 Approximately 80% of patients with PE have lower extremity deep venous thrombosis (DVT). Conversely, PE occurs in up to 50% of patients with proximal DVT.
Approximately 80% of patients with PE have lower extremity deep venous thrombosis (DVT). Conversely, PE occurs in up to 50% of patients with proximal DVT.
 Risk factors include surgery, immobility, hypercoaguability, and age.
Risk factors include surgery, immobility, hypercoaguability, and age.
 D-dimer testing is highly sensitive for DVT and PE but nonspecific; D-dimer elevation occurs with infection, cancer, trauma, and other inflammatory states.
D-dimer testing is highly sensitive for DVT and PE but nonspecific; D-dimer elevation occurs with infection, cancer, trauma, and other inflammatory states.
 Treatment usually consists of anticoagulation with heparin or low-molecular-weight heparin, followed by warfarin for at least 3 to 6 months. If an individual harbors a contraindication to anticoagulation, an inferior vena cava (IVC) filter can be inserted.
Treatment usually consists of anticoagulation with heparin or low-molecular-weight heparin, followed by warfarin for at least 3 to 6 months. If an individual harbors a contraindication to anticoagulation, an inferior vena cava (IVC) filter can be inserted.
 Acute treatment of PE with intravenous thrombolytic agents has not yet definitively demonstrated advantages over long-term anticoagulation and is usually reserved for very acutely ill patients in order to decrease their clot burden.
Acute treatment of PE with intravenous thrombolytic agents has not yet definitively demonstrated advantages over long-term anticoagulation and is usually reserved for very acutely ill patients in order to decrease their clot burden.
Radiologic
 Most patients with PE have abnormal chest radiographs, but the findings are nonspecific—for example, either or both atelectasis and pulmonary parenchymal opacities. However, in the appropriate circumstances, a normal radiograph is actually most helpful in assessment for PE. A normal chest radiograph in a patient with dyspnea and hypoxia (PaO2 ≤ 70 mm Hg) should strongly raise the possibility of PE, because it effectively excludes other entities that clinically mimic PE. The other main function of the chest radiograph is to aid in interpretation of the ventilation-perfusion imaging in a patient with suspected PE (see Chapter 10, Case 17).
Most patients with PE have abnormal chest radiographs, but the findings are nonspecific—for example, either or both atelectasis and pulmonary parenchymal opacities. However, in the appropriate circumstances, a normal radiograph is actually most helpful in assessment for PE. A normal chest radiograph in a patient with dyspnea and hypoxia (PaO2 ≤ 70 mm Hg) should strongly raise the possibility of PE, because it effectively excludes other entities that clinically mimic PE. The other main function of the chest radiograph is to aid in interpretation of the ventilation-perfusion imaging in a patient with suspected PE (see Chapter 10, Case 17).
 CTA and ventilation-perfusion scintigraphy have become the first-line imaging techniques for the evaluation for PE.
CTA and ventilation-perfusion scintigraphy have become the first-line imaging techniques for the evaluation for PE.
 Advantages of CTA include noninvasive nature, short imaging time, ability to identify other etiologies that may be responsible for symptoms, and detection of right-heart strain in the setting of massive PE. Disadvantages include a substantial radiation dose (of particular concern, the breasts in young females), sensitivity to motion artifact, and need for use of iodinated contrast.
Advantages of CTA include noninvasive nature, short imaging time, ability to identify other etiologies that may be responsible for symptoms, and detection of right-heart strain in the setting of massive PE. Disadvantages include a substantial radiation dose (of particular concern, the breasts in young females), sensitivity to motion artifact, and need for use of iodinated contrast.
 Conventional pulmonary angiography is now rarely performed for PE diagnosis, given the excellent noninvasive imaging options. Vascular access is usually from the common femoral vein, although the internal jugular and brachial vein can also be used to gain access. Selective angiography is usually performed with the pigtail-type catheter placed into the right or left pulmonary artery.
Conventional pulmonary angiography is now rarely performed for PE diagnosis, given the excellent noninvasive imaging options. Vascular access is usually from the common femoral vein, although the internal jugular and brachial vein can also be used to gain access. Selective angiography is usually performed with the pigtail-type catheter placed into the right or left pulmonary artery.
 The presence of occlusive or nearly occlusive intra-arterial filling defects on pulmonary angiography is diagnostic of acute PE. Complete arterial occlusion (so-called vessel cutoff) is also highly suggestive of the diagnosis. Linear filling defects, luminal narrowing, and occluded vessels with a smooth meniscus or tapered margin suggest chronic, rather than acute, PE.
The presence of occlusive or nearly occlusive intra-arterial filling defects on pulmonary angiography is diagnostic of acute PE. Complete arterial occlusion (so-called vessel cutoff) is also highly suggestive of the diagnosis. Linear filling defects, luminal narrowing, and occluded vessels with a smooth meniscus or tapered margin suggest chronic, rather than acute, PE.
 Catheter-directed thrombolytic therapy and mechanical fragmentation have been shown to be relatively safe and effective means of treatment of massive PE with hemodynamic compromise.
Catheter-directed thrombolytic therapy and mechanical fragmentation have been shown to be relatively safe and effective means of treatment of massive PE with hemodynamic compromise.
SUGGESTED READING
Konstantinides S. Clinical practice. Acute pulmonary embolism. N Engl J Med 2008;359(26):2804–2813.
Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol 2009;20(11):1431–1440.
Remy-Jardin M, Pistolesi M, Goodman LR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology 2007;245(2):315–329.
Tapson VF. Acute pulmonary embolism. N Engl J Med 2008;358(10):1037–1052.
Young T, Tang H, Aukes J, Hughes R. Vena caval filters for the prevention of pulmonary embolism. Cochrane Database Syst Rev 2007;(4):CD006212.
DANIEL J.
STACKHOUSE
AND
CHARLES Y.
KIM
HISTORY
A 51-year-old man with bright red blood per rectum.
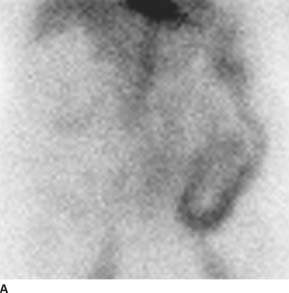
 FIGURE 7-3A A Tc99-labeled red blood cell nuclear medicine study shows radiotracer uptake throughout the sigmoid colon and left colon, consistent with active lower GI bleeding.
FIGURE 7-3A A Tc99-labeled red blood cell nuclear medicine study shows radiotracer uptake throughout the sigmoid colon and left colon, consistent with active lower GI bleeding.
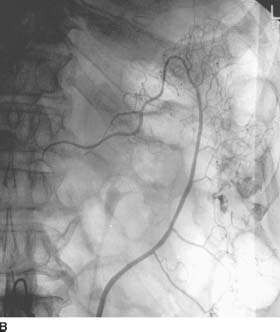
 FIGURE 7-3B Arteriogram of the IMA shows a bleeding site in the descending colon with extravasation of contrast material into the bowel. No abnormal vascularity to suggest tumor vessels or early draining veins to suggest angiodysplasia is seen.
FIGURE 7-3B Arteriogram of the IMA shows a bleeding site in the descending colon with extravasation of contrast material into the bowel. No abnormal vascularity to suggest tumor vessels or early draining veins to suggest angiodysplasia is seen.
DIFFERENTIAL DIAGNOSIS
 The following is a differential diagnosis for hemorrhage from a left colon bleeding site (as in the case shown):
The following is a differential diagnosis for hemorrhage from a left colon bleeding site (as in the case shown):
 Diverticulosis: This entity is the most common cause of colorectal bleeding. Diverticula occur more commonly in the left colon; those that occur in the right colon actually carry a higher risk of bleeding. This entity does not have a characteristic imaging feature. Given the lack of specific imaging features as described in these other differential diagnoses, this is statistically the most likely diagnosis.
Diverticulosis: This entity is the most common cause of colorectal bleeding. Diverticula occur more commonly in the left colon; those that occur in the right colon actually carry a higher risk of bleeding. This entity does not have a characteristic imaging feature. Given the lack of specific imaging features as described in these other differential diagnoses, this is statistically the most likely diagnosis.
 Angiodysplasia: This acquired lesion accounts for up to 20% of cases of colorectal bleeding. The angiographic appearance is that of a small tuft of vessels with at least one early draining vein. These findings are not seen in the case illustrated and therefore this diagnosis is unlikely.
Angiodysplasia: This acquired lesion accounts for up to 20% of cases of colorectal bleeding. The angiographic appearance is that of a small tuft of vessels with at least one early draining vein. These findings are not seen in the case illustrated and therefore this diagnosis is unlikely.
 Neoplasm: Tumors account for about 10% of cases of colorectal bleeding. They typically are seen to have a focal parenchymal blush and tumor neovascularity at angiography, which are not present in this case. Therefore, this diagnosis is unlikely. Nonetheless, angiography cannot definitively exclude a neoplasm. Instead, endoscopy is typically performed.
Neoplasm: Tumors account for about 10% of cases of colorectal bleeding. They typically are seen to have a focal parenchymal blush and tumor neovascularity at angiography, which are not present in this case. Therefore, this diagnosis is unlikely. Nonetheless, angiography cannot definitively exclude a neoplasm. Instead, endoscopy is typically performed.
 Colitis: This entity accounts for 5% to 10% of cases of colorectal bleeding. At angiography, prominent opacification of involved segments of colon is typically seen during the capillary phase, which is not present in this case. Therefore, this diagnosis is unlikely.
Colitis: This entity accounts for 5% to 10% of cases of colorectal bleeding. At angiography, prominent opacification of involved segments of colon is typically seen during the capillary phase, which is not present in this case. Therefore, this diagnosis is unlikely.
Active hemorrhage due to diverticulosis in the descending colon
KEY FACTS
Clinical
 Prompt and adequate hemodynamic stabilization (e.g., placement of large-bore intravenous lines and vascular repletion) of the patient with gastrointestinal (GI) hemorrhage is imperative before diagnostic procedures are started.
Prompt and adequate hemodynamic stabilization (e.g., placement of large-bore intravenous lines and vascular repletion) of the patient with gastrointestinal (GI) hemorrhage is imperative before diagnostic procedures are started.
 Eighty percent of patients with GI bleeding have spontaneous resolution of the hemorrhage without treatment.
Eighty percent of patients with GI bleeding have spontaneous resolution of the hemorrhage without treatment.
 It is important to determine whether the source of bleeding is from the upper (i.e., above the ligament of Treitz) or lower GI tract. If gastric lavage contents are clear, the source is presumed to be distal to the pylorus. Otherwise, if positive, the source is most likely in the upper GI tract.
It is important to determine whether the source of bleeding is from the upper (i.e., above the ligament of Treitz) or lower GI tract. If gastric lavage contents are clear, the source is presumed to be distal to the pylorus. Otherwise, if positive, the source is most likely in the upper GI tract.
 Upper GI tract bleeding is five times more common than lower GI tract bleeding.
Upper GI tract bleeding is five times more common than lower GI tract bleeding.
 In cases of upper GI hemorrhage, endoscopy will frequently identify the bleeding source and provide the initial means of treatment, including cautery, injection of epinephrine, or in the case of variceal bleeding, sclerotherapy or banding. Endoscopy has a lower diagnostic and therapeutic yield for lower GI hemorrhage.
In cases of upper GI hemorrhage, endoscopy will frequently identify the bleeding source and provide the initial means of treatment, including cautery, injection of epinephrine, or in the case of variceal bleeding, sclerotherapy or banding. Endoscopy has a lower diagnostic and therapeutic yield for lower GI hemorrhage.
 The most common source of massive bleeding from the upper GI tract is ulceration. Other causes of upper GI tract bleeding include gastritis, variceal bleeding, Dieulafoy lesion (i.e., a large tortuous arteriole within the stomach wall), tumor, Mallory-Weiss tear, and angiodyplasia.
The most common source of massive bleeding from the upper GI tract is ulceration. Other causes of upper GI tract bleeding include gastritis, variceal bleeding, Dieulafoy lesion (i.e., a large tortuous arteriole within the stomach wall), tumor, Mallory-Weiss tear, and angiodyplasia.
 Sources of small bowel hemorrhage include ulceration, diverticula, enteritis, neoplasm, angiodysplasia, and aortoenteric fistula.
Sources of small bowel hemorrhage include ulceration, diverticula, enteritis, neoplasm, angiodysplasia, and aortoenteric fistula.
 Sources of colonic hemorrhage include diverticular disease, angiodysplasia, neoplasm, and colitis. The presence of bright red blood per rectum can be a false-localizing sign for colonic hemorrhage because the upper GI tract is the source of hemorrhage in up to 10% of patients with such bleeding.
Sources of colonic hemorrhage include diverticular disease, angiodysplasia, neoplasm, and colitis. The presence of bright red blood per rectum can be a false-localizing sign for colonic hemorrhage because the upper GI tract is the source of hemorrhage in up to 10% of patients with such bleeding.
 If a patient is suspected to be actively bleeding, tagged red blood cell scintigraphy, which can detect bleeding at a rate of 0.1 mL/min, can be performed to determine whether the patient is actively bleeding and to attempt to localize the site of bleeding.
If a patient is suspected to be actively bleeding, tagged red blood cell scintigraphy, which can detect bleeding at a rate of 0.1 mL/min, can be performed to determine whether the patient is actively bleeding and to attempt to localize the site of bleeding.
Radiologic
 Indications for urgent mesenteric angiography include massive nonvariceal GI bleeding (from either an upper GI or lower GI source) for which the bleeding site either cannot be identified or cannot be treated via endoscopic means.
Indications for urgent mesenteric angiography include massive nonvariceal GI bleeding (from either an upper GI or lower GI source) for which the bleeding site either cannot be identified or cannot be treated via endoscopic means.
 Angiography can detect active hemorrhage only if the rate of bleeding is at least 0.5 to 1.0 mL/min, a much more vigorous rate than that detectable by nuclear scintigraphy.
Angiography can detect active hemorrhage only if the rate of bleeding is at least 0.5 to 1.0 mL/min, a much more vigorous rate than that detectable by nuclear scintigraphy.
 Angiographic findings include active extravasation of contrast material into the bowel lumen, a pseudoaneurysm, an early draining vein in the setting of angiodysplasia, or a hypervascular mass.
Angiographic findings include active extravasation of contrast material into the bowel lumen, a pseudoaneurysm, an early draining vein in the setting of angiodysplasia, or a hypervascular mass.
 For upper GI bleeding, selective injections of the celiac, left gastric, gastroduodenal, and superior mesenteric arteries (SMAs) are performed routinely.
For upper GI bleeding, selective injections of the celiac, left gastric, gastroduodenal, and superior mesenteric arteries (SMAs) are performed routinely.
 For lower GI bleeding, injection of the SMA and inferior mesenteric artery (IMA) are routinely performed. If endoscopy or scintigraphy provides localizing information, selective injections are often performed.
For lower GI bleeding, injection of the SMA and inferior mesenteric artery (IMA) are routinely performed. If endoscopy or scintigraphy provides localizing information, selective injections are often performed.
 If a site of contrast material extravasation is identified, attempts should be made to advance a microcatheter as distal as possible toward the site of bleeding. Embolization should then be performed using coils, particles, or gelatin sponge pledgets.
If a site of contrast material extravasation is identified, attempts should be made to advance a microcatheter as distal as possible toward the site of bleeding. Embolization should then be performed using coils, particles, or gelatin sponge pledgets.
 Vasopressin infusion, which was previously the mainstay of treatment of lower GI bleeding, is currently performed only when a microcatheter cannot be advanced into an appropriate distal artery.
Vasopressin infusion, which was previously the mainstay of treatment of lower GI bleeding, is currently performed only when a microcatheter cannot be advanced into an appropriate distal artery.
 For upper GI bleeding, if extravasation of contrast material is not identified, empirical embolization of the gastroduodenal artery or left gastric artery is often effective when a site of suspected bleeding is seen at endoscopy.
For upper GI bleeding, if extravasation of contrast material is not identified, empirical embolization of the gastroduodenal artery or left gastric artery is often effective when a site of suspected bleeding is seen at endoscopy.
 The risk of ischemia is relatively small given the rich collateral supply to the GI tract; the risk is higher in the lower GI tract.
The risk of ischemia is relatively small given the rich collateral supply to the GI tract; the risk is higher in the lower GI tract.
SUGGESTED READING
Burke SJ, Golzarian J, Weldon D, Sun S. Nonvariceal upper gastrointestinal bleeding. Eur Radiol 2007;17(7):1714–1726.
Edelman DA, Sugawa C. Lower gastrointestinal bleeding: a review. Surg Endosc 2007;21(4):514–520.
Hastings GS. Angiographic localization and transcatheter treatment of gastrointestinal bleeding. Radiographics 2000;20(4):1160–1168.
Millward SF. ACR Appropriateness criteria on treatment of acute nonvariceal gastrointestinal tract bleeding. J Am Coll Radiol 2008;5(4):550–554.
Walker TG. Acute gastrointestinal hemorrhage. Tech Vasc Interv Radiol 2009;12(2):80–91.
PAUL V.
SUHOCKI
AND
CHARLES Y.
KIM
HISTORY
A 58-year-old woman who was struck in the chest during a motor vehicle accident.
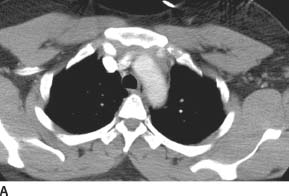
 FIGURE 7-4A Axial contrast-enhanced CT through the aortic arch shows a soft tissue density in the anterior mediastinum, which is in continuity with the aortic wall.
FIGURE 7-4A Axial contrast-enhanced CT through the aortic arch shows a soft tissue density in the anterior mediastinum, which is in continuity with the aortic wall.
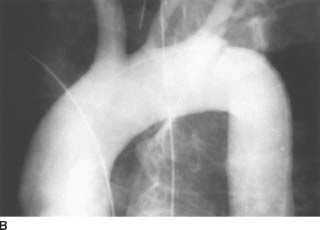
 FIGURE 7-4B Aortogram shows a linear lucency extending inferiorly from the left upper border of the aorta, just beyond the origin of the left subclavian artery. In this same region, there is a discontinuity (“step off”) in the superior contour of the aortic lumen. These findings represent aortic injury.
FIGURE 7-4B Aortogram shows a linear lucency extending inferiorly from the left upper border of the aorta, just beyond the origin of the left subclavian artery. In this same region, there is a discontinuity (“step off”) in the superior contour of the aortic lumen. These findings represent aortic injury.
DIFFERENTIAL DIAGNOSIS
 Mediastinal hematoma due to aortic injury: The soft tissue density in the anterior mediastinum on the CT image is contiguous with the aortic wall, raising the possibility of hemorrhage due to an aortic tear. The abnormalities seen on the aortogram are consistent with injury to the aorta just distal to the left subclavian artery origin, and are an indication for emergent surgical repair.
Mediastinal hematoma due to aortic injury: The soft tissue density in the anterior mediastinum on the CT image is contiguous with the aortic wall, raising the possibility of hemorrhage due to an aortic tear. The abnormalities seen on the aortogram are consistent with injury to the aorta just distal to the left subclavian artery origin, and are an indication for emergent surgical repair.
 Mediastinal hematoma due to venous bleeding: The most common cause of mediastinal hematoma is venous hemorrhage, which can be confidently diagnosed if a distinct fat plane exists between the hematoma and the aortic wall. However, in the case shown here, the hematoma is contiguous with the aorta. Furthermore, the aortogram shows findings indicative of an arterial injury. Venous injury could certainly occur concurrently with arterial injury in the setting of blunt trauma. However, the diagnosis of venous hemorrhage becomes irrelevant when arterial injury is present, and is therefore the wrong diagnosis.
Mediastinal hematoma due to venous bleeding: The most common cause of mediastinal hematoma is venous hemorrhage, which can be confidently diagnosed if a distinct fat plane exists between the hematoma and the aortic wall. However, in the case shown here, the hematoma is contiguous with the aorta. Furthermore, the aortogram shows findings indicative of an arterial injury. Venous injury could certainly occur concurrently with arterial injury in the setting of blunt trauma. However, the diagnosis of venous hemorrhage becomes irrelevant when arterial injury is present, and is therefore the wrong diagnosis.
 Residual thymus: This diagnosis might be considered on the basis of the finding of a soft tissue density in the anterior mediastinum. However, the thymus gland reaches its peak size during puberty and gradually involutes until it is essentially replaced by fat during the fourth decade (i.e., at an age much younger than the patient in the case shown here). Furthermore, this diagnosis would not account for the angiographic findings; this diagnosis is incorrect.
Residual thymus: This diagnosis might be considered on the basis of the finding of a soft tissue density in the anterior mediastinum. However, the thymus gland reaches its peak size during puberty and gradually involutes until it is essentially replaced by fat during the fourth decade (i.e., at an age much younger than the patient in the case shown here). Furthermore, this diagnosis would not account for the angiographic findings; this diagnosis is incorrect.
 Lymphadenopathy: The mass in the anterior mediastinum could, in theory, represent confluent lymphadenopathy. However, additional enlarged lymph nodes would be expected; none are present in the CT scan shown here, making this diagnosis unlikely. Furthermore, lymphadenopathy would not explain the angiographic findings. This diagnosis is incorrect.
Lymphadenopathy: The mass in the anterior mediastinum could, in theory, represent confluent lymphadenopathy. However, additional enlarged lymph nodes would be expected; none are present in the CT scan shown here, making this diagnosis unlikely. Furthermore, lymphadenopathy would not explain the angiographic findings. This diagnosis is incorrect.
DIAGNOSIS
Traumatic tear at the isthmus of the thoracic aorta with associated mediastinal hematoma
Clinical
 Only 10% to 20% of patients with traumatic rupture of the aorta survive long enough to be treated at a hospital. Of those, the mortality rate of untreated aortic rupture is 95% to 97%.
Only 10% to 20% of patients with traumatic rupture of the aorta survive long enough to be treated at a hospital. Of those, the mortality rate of untreated aortic rupture is 95% to 97%.
 The mechanism of injury is most commonly motor vehicle collisions, followed by falls from height, pedestrian-automobile collisions, and crush injuries.
The mechanism of injury is most commonly motor vehicle collisions, followed by falls from height, pedestrian-automobile collisions, and crush injuries.
 Based on autopsy series, the majority of aortic injuries are located at the isthmus (within 2 cm of the origin of the left subclavian artery). This is the site at which the ligamentum arteriosum attaches the aorta to the left pulmonary artery. The isthmus is the site of aortic laceration in the vast majority (i.e., 90% to 95%) of patients who survive to reach the hospital.
Based on autopsy series, the majority of aortic injuries are located at the isthmus (within 2 cm of the origin of the left subclavian artery). This is the site at which the ligamentum arteriosum attaches the aorta to the left pulmonary artery. The isthmus is the site of aortic laceration in the vast majority (i.e., 90% to 95%) of patients who survive to reach the hospital.
 Approximately 10% of aortic injuries involve the ascending aorta, which is nearly always fatal.
Approximately 10% of aortic injuries involve the ascending aorta, which is nearly always fatal.
 Less commonly involved sites are the aortic arch and the distal thoracic, diaphragmatic, and abdominal aortic segments.
Less commonly involved sites are the aortic arch and the distal thoracic, diaphragmatic, and abdominal aortic segments.
 Emergency surgical repair, usually by placement of a prosthetic aortic graft, is typically indicated. Primary repair (i.e., suturing of the laceration) and patch angioplasty are other, less frequently used, procedures.
Emergency surgical repair, usually by placement of a prosthetic aortic graft, is typically indicated. Primary repair (i.e., suturing of the laceration) and patch angioplasty are other, less frequently used, procedures.
Radiologic
 Chest radiography is neither sensitive nor specific for aortic injury, but is frequently obtained in trauma patients. A number of chest radiographic findings should strongly raise suspicion of an aortic tear in a trauma patient: (1) widened mediastinum, (2) depressed left mainstem bronchus, (3) deviation of the esophagus (deviated nasogastric tube) and trachea at the level of the T4 vertebral body, (4) obscured margins of the aortic arch, (5) left apical opacity (so-called apical cap), (6) fracture of any of the first three ribs, (7) widening of a paraspinal line, and (8) widening of the right paratracheal stripe.
Chest radiography is neither sensitive nor specific for aortic injury, but is frequently obtained in trauma patients. A number of chest radiographic findings should strongly raise suspicion of an aortic tear in a trauma patient: (1) widened mediastinum, (2) depressed left mainstem bronchus, (3) deviation of the esophagus (deviated nasogastric tube) and trachea at the level of the T4 vertebral body, (4) obscured margins of the aortic arch, (5) left apical opacity (so-called apical cap), (6) fracture of any of the first three ribs, (7) widening of a paraspinal line, and (8) widening of the right paratracheal stripe.
 A negative chest radiograph should not preclude further radiologic evaluation, which should be prompted, based on the mechanism of trauma and clinical suspicion of aortic injury.
A negative chest radiograph should not preclude further radiologic evaluation, which should be prompted, based on the mechanism of trauma and clinical suspicion of aortic injury.
 CTA is currently considered the diagnostic test of choice for evaluation of aortic injury, having very high sensitivity and specificity. The advent of multidetector CT and EKG-gating have greatly improved imaging quality of the proximal ascending aorta.
CTA is currently considered the diagnostic test of choice for evaluation of aortic injury, having very high sensitivity and specificity. The advent of multidetector CT and EKG-gating have greatly improved imaging quality of the proximal ascending aorta.
 Direct CTA findings of acute aortic injury include an intimal flap, pseudoaneurysm (i.e., a contained rupture), intraluminal mural thrombus, abnormal aortic contour, and sudden change in aortic caliber. Any one of these findings is considered definitive for diagnosis and no additional imaging is necessary. Surgical consultation should be performed expeditiously.
Direct CTA findings of acute aortic injury include an intimal flap, pseudoaneurysm (i.e., a contained rupture), intraluminal mural thrombus, abnormal aortic contour, and sudden change in aortic caliber. Any one of these findings is considered definitive for diagnosis and no additional imaging is necessary. Surgical consultation should be performed expeditiously.
 The presence of a mediastinal hematoma is considered an indirect finding, and its significance depends on its location. Separation of the hematoma from the aorta by an intact fat plane is an indication that the hematoma is not due to aortic injury but instead is typically due to injury of small mediastinal veins. If the hematoma is truly periaortic, then further evaluation is warranted, since this could represent a rare occult aortic injury versus injury of small periaortic veins or the vasa vasorum.
The presence of a mediastinal hematoma is considered an indirect finding, and its significance depends on its location. Separation of the hematoma from the aorta by an intact fat plane is an indication that the hematoma is not due to aortic injury but instead is typically due to injury of small mediastinal veins. If the hematoma is truly periaortic, then further evaluation is warranted, since this could represent a rare occult aortic injury versus injury of small periaortic veins or the vasa vasorum.
 The rate of false negative CTA studies for aortic tear is very low. Nonetheless, the presence of a truly periaortic mediastinal hematoma is an indication for further imaging. Further evaluation could be performed with transesophageal echoaortography, intravascular ultrasound, conventional angiography, or follow-up CTA after 2 to 3 days, if the patient is stable.
The rate of false negative CTA studies for aortic tear is very low. Nonetheless, the presence of a truly periaortic mediastinal hematoma is an indication for further imaging. Further evaluation could be performed with transesophageal echoaortography, intravascular ultrasound, conventional angiography, or follow-up CTA after 2 to 3 days, if the patient is stable.
 On CTA, a careful search for branch vessel injury should be performed in the presence of mediastinal hematoma.
On CTA, a careful search for branch vessel injury should be performed in the presence of mediastinal hematoma.
 Conventional angiography for the diagnosis of acute aortic injury has taken on a substantially limited role in the multidetector CT era, primarily due to the time delay it imposes until treatment. However, it remains a valuable technique for problem solving in the stable patient, for planning prior to endovascular stent graft therapy, and detection of branch vessel injury. During aortography, an injection in the left anterior oblique projection should first be performed, followed by a right anterior oblique or lateral projection. The brachiocephalic vessels and region of the descending aorta above the celiac artery should be included in the field of view. An aortic laceration can be missed if only a single projection is used at aortography.
Conventional angiography for the diagnosis of acute aortic injury has taken on a substantially limited role in the multidetector CT era, primarily due to the time delay it imposes until treatment. However, it remains a valuable technique for problem solving in the stable patient, for planning prior to endovascular stent graft therapy, and detection of branch vessel injury. During aortography, an injection in the left anterior oblique projection should first be performed, followed by a right anterior oblique or lateral projection. The brachiocephalic vessels and region of the descending aorta above the celiac artery should be included in the field of view. An aortic laceration can be missed if only a single projection is used at aortography.
 Most patients with aortic tear develop a pseudoaneurysm at the site of laceration. Solely an intimal tear, seen as a linear lucency on the angiogram, is present in 5% to 10% of cases.
Most patients with aortic tear develop a pseudoaneurysm at the site of laceration. Solely an intimal tear, seen as a linear lucency on the angiogram, is present in 5% to 10% of cases.
 Causes of false-positive aortograms, seen in 1% of cases, include ductus diverticulum, aortic aneurysm, atherosclerotic plaque, and artifacts due to inflow of unopacified blood (i.e., “streaming” artifacts).
Causes of false-positive aortograms, seen in 1% of cases, include ductus diverticulum, aortic aneurysm, atherosclerotic plaque, and artifacts due to inflow of unopacified blood (i.e., “streaming” artifacts).
 Other valuable diagnostic techniques for diagnosis of aortic tear include transesophageal echoaortography and intravascular sonography.
Other valuable diagnostic techniques for diagnosis of aortic tear include transesophageal echoaortography and intravascular sonography.
 Although surgical repair has been considered to be the standard of care for aortic tear, a growing body of literature supports the use of endovascular stent grafting.
Although surgical repair has been considered to be the standard of care for aortic tear, a growing body of literature supports the use of endovascular stent grafting.
SUGGESTED READING
Alkadhi H, Wildermuth S, Desbiolles L, et al. Vascular emergencies of the thorax after blunt and iatrogenic trauma: multi-detector row CT and three-dimensional imaging. Radiographics 2004;24(5):1239–1255.
Feczko JD, Lynch L, Pless JE, et al. An autopsy case review of 142 nonpenetrating (blunt) injuries of the aorta. J Trauma 1992;33:846–849.
Malloy PC, Richard HM III. Thoracic angiography and intervention in trauma. Radiol Clin North Am 2006;44(2):239–249, viii.
Steenburg SD, Ravenel JG, Ikonomidis JS, et al. Acute traumatic aortic injury: imaging evaluation and management. Radiology 2008;248(3):748–762.
Walsh SR, Tang TY, Sadat U, et al. Endovascular stenting versus open surgery for thoracic aortic disease: systematic review and meta-analysis of perioperative results. J Vasc Surg 2008;47(5):1094–1098.
CYNTHIA S.
PAYNE
AND
CHARLES Y.
KIM
HISTORY
A 58-year-old woman with substernal pleuritic chest pain and new dyspnea on exertion. She subsequently experienced an episode of transient hemiplegia following infusion of an intravenous catheter with saline.
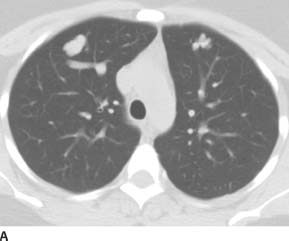
 FIGURE 7-5A Axial unenhanced CT image a lobulated solid-appearing lesion in the periphery of each lung. Both lesions appear to have prominent connecting vessels, which are suspicious for associated draining pulmonary veins (confirmed on adjacent images which are not shown).
FIGURE 7-5A Axial unenhanced CT image a lobulated solid-appearing lesion in the periphery of each lung. Both lesions appear to have prominent connecting vessels, which are suspicious for associated draining pulmonary veins (confirmed on adjacent images which are not shown).
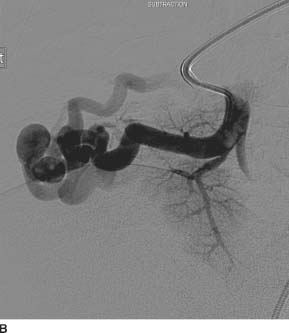
 FIGURE 7-5B Selective angiogram of a right lower lobe pulmonary artery shows an enlarged and tortuous pulmonary artery branch supplying a vascular nidus with a prominent early draining vein.
FIGURE 7-5B Selective angiogram of a right lower lobe pulmonary artery shows an enlarged and tortuous pulmonary artery branch supplying a vascular nidus with a prominent early draining vein.
 Malignancy: Based on the CT image, malignancy should always be considered for multiple pulmonary lesions. However, the vascular nature as demonstrated on angiography is not compatible with a solid tumor.
Malignancy: Based on the CT image, malignancy should always be considered for multiple pulmonary lesions. However, the vascular nature as demonstrated on angiography is not compatible with a solid tumor.
 Hamartoma: This diagnosis might be considered because of the presence of lobulated, well-circumscribed lesions in the periphery of the lung. However, the lesions shown in the CT image lack the characteristic “popcorn” calcifications and macroscopic fat on CT typical of hamartomas. Given the lack of these findings and the highly vascular nature of the lesions as seen on angiography, this diagnosis is unlikely.
Hamartoma: This diagnosis might be considered because of the presence of lobulated, well-circumscribed lesions in the periphery of the lung. However, the lesions shown in the CT image lack the characteristic “popcorn” calcifications and macroscopic fat on CT typical of hamartomas. Given the lack of these findings and the highly vascular nature of the lesions as seen on angiography, this diagnosis is unlikely.
 Granuloma: The CT finding of multiple well-circumscribed lesions may suggest granulomatous disease related to tuberculous or fungal infection on CT. However, the large size and multilobulated configuration of the lesions on CT would be unusual for granulomatous disease. Furthermore, the angiographic findings are not consistent with this diagnosis.
Granuloma: The CT finding of multiple well-circumscribed lesions may suggest granulomatous disease related to tuberculous or fungal infection on CT. However, the large size and multilobulated configuration of the lesions on CT would be unusual for granulomatous disease. Furthermore, the angiographic findings are not consistent with this diagnosis.
 Pulmonary arteriovenous malformation (AVM): The lobulated appearance on CT with associated draining veins is characteristic of pulmonary AVM. This diagnosis is definitively established on the pulmonary angiogram that shows an enlarged feeding artery, a vascular nidus, and enlarged early draining veins. The multiplicity of lesions in the case shown here should strongly suggest a diagnosis of hereditary hemorrhagic telangiectasia (HHT).
Pulmonary arteriovenous malformation (AVM): The lobulated appearance on CT with associated draining veins is characteristic of pulmonary AVM. This diagnosis is definitively established on the pulmonary angiogram that shows an enlarged feeding artery, a vascular nidus, and enlarged early draining veins. The multiplicity of lesions in the case shown here should strongly suggest a diagnosis of hereditary hemorrhagic telangiectasia (HHT).
DIAGNOSIS
Pulmonary arteriovenous malformations in the setting of HHT
KEY FACTS
Clinical
 Pulmonary arteriovenous malformations may receive arterial supply from only one arterial source (by definition termed an AV fistula) or from multiple feeding arteries (termed an AVM). Approximately 80% are AV fistulas. For the purposes of this review, the term AVM will be used to refer to both of these entities.
Pulmonary arteriovenous malformations may receive arterial supply from only one arterial source (by definition termed an AV fistula) or from multiple feeding arteries (termed an AVM). Approximately 80% are AV fistulas. For the purposes of this review, the term AVM will be used to refer to both of these entities.
 Patients can present with hemoptysis, chest pain, hypoxia, and neurologic symptoms (including stroke, transient ischemic attacks, and cerebral abscess formation) from paradoxical emboli, since the AVM effectively serves as a right to left shunt.
Patients can present with hemoptysis, chest pain, hypoxia, and neurologic symptoms (including stroke, transient ischemic attacks, and cerebral abscess formation) from paradoxical emboli, since the AVM effectively serves as a right to left shunt.
 Clinical signs can include digital clubbing, cyanosis, polycythemia, chest bruit, and neurologic symptoms and signs due to paradoxical emboli.
Clinical signs can include digital clubbing, cyanosis, polycythemia, chest bruit, and neurologic symptoms and signs due to paradoxical emboli.
 Pulmonary AVMs are rarely symptomatic if <2 cm in size. Many investigators maintain that treatment is warranted even for asymptomatic lesions <2 cm in size or those having a feeding artery with a diameter of >3 mm.
Pulmonary AVMs are rarely symptomatic if <2 cm in size. Many investigators maintain that treatment is warranted even for asymptomatic lesions <2 cm in size or those having a feeding artery with a diameter of >3 mm.
 Approximately 15% of pulmonary AVMs occur sporadically, occasionally associated with prior trauma, infection, hepatopulmonary syndrome, or surgery. Approximately 85% occur as part of HHT (also known as Osler-Weber-Rendu syndrome). HHT is an autosomal dominant condition with variable penetrance and is characteristically associated with the triad of epistaxis, telangiectasias, and family history of the syndrome. Approximately 15% to 25% of patients with HHT have pulmonary AVMs.
Approximately 15% of pulmonary AVMs occur sporadically, occasionally associated with prior trauma, infection, hepatopulmonary syndrome, or surgery. Approximately 85% occur as part of HHT (also known as Osler-Weber-Rendu syndrome). HHT is an autosomal dominant condition with variable penetrance and is characteristically associated with the triad of epistaxis, telangiectasias, and family history of the syndrome. Approximately 15% to 25% of patients with HHT have pulmonary AVMs.
Radiologic
 More than 95% of patients with pulmonary AVMs have an abnormal chest radiograph.
More than 95% of patients with pulmonary AVMs have an abnormal chest radiograph.
 Approximately two-thirds of pulmonary AVMs are solitary lesions.
Approximately two-thirds of pulmonary AVMs are solitary lesions.
 High-resolution CT has an extremely high sensitivity for detecting the presence of a pulmonary AVM, in which the enlarged draining vein is easily visualized. CTA and MRA can help confirm the vascular nature of the lesion. Arterial phase CTA images or time-resolved MRA can confirm the early arterial filling, which is diagnostic of an AVM.
High-resolution CT has an extremely high sensitivity for detecting the presence of a pulmonary AVM, in which the enlarged draining vein is easily visualized. CTA and MRA can help confirm the vascular nature of the lesion. Arterial phase CTA images or time-resolved MRA can confirm the early arterial filling, which is diagnostic of an AVM.
 Renal and cerebral radiotracer activity during lung perfusion study is a characteristic finding that can be seen due to the right-to-left shunting resulting from pulmonary AVMs. The presence of these findings during a lung perfusion study is a clue to the diagnosis of pulmonary AVM.
Renal and cerebral radiotracer activity during lung perfusion study is a characteristic finding that can be seen due to the right-to-left shunting resulting from pulmonary AVMs. The presence of these findings during a lung perfusion study is a clue to the diagnosis of pulmonary AVM.
 Pulmonary angiography can confirm the diagnosis, classify the type of lesion, and provide the means for endovascular treatment.
Pulmonary angiography can confirm the diagnosis, classify the type of lesion, and provide the means for endovascular treatment.
 Treatment by permanent occlusion with coils or other embolic devices has a rate of high success with low morbidity and low mortality. The major risk to be considered during the procedure is unintentional embolization of the systemic circulation by the coil or balloon during deployment. In addition, particular care must be taken to avoid presence of air bubbles, which could enter the cerebral circulation during catheter flushing and angiography.
Treatment by permanent occlusion with coils or other embolic devices has a rate of high success with low morbidity and low mortality. The major risk to be considered during the procedure is unintentional embolization of the systemic circulation by the coil or balloon during deployment. In addition, particular care must be taken to avoid presence of air bubbles, which could enter the cerebral circulation during catheter flushing and angiography.
SUGGESTED READING
Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med J 2002;78(918):191–197.
Pollak JS, Saluja S, Thabet A, et al. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol 2006;17(1):35–44.
Remy-Jardin M, Wattinne L, Remy J. Transcatheter occlusion of pulmonary arterial circulation and collateral supply: failures, incidents, and complications. Radiology 1991;180:699–705.
DANIEL J.
STACKHOUSE
HISTORY
A 36-year-old man with fever, hematuria, and elevated sedimentation rate. He has a history of hepatitis B infection.
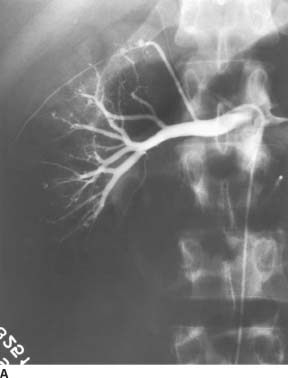
 FIGURE 7-6A Renal arteriogram shows multiple distal microaneurysms and distal arterial stenoses.
FIGURE 7-6A Renal arteriogram shows multiple distal microaneurysms and distal arterial stenoses.
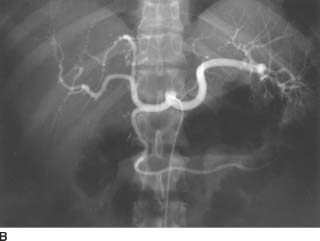
 FIGURE 7-6B Celiac arteriogram also shows multiple microaneurysms and distal arterial stenoses.
FIGURE 7-6B Celiac arteriogram also shows multiple microaneurysms and distal arterial stenoses.
 Polyarteritis nodosa (PAN): PAN is a necrotizing vasculitis of unknown etiology, which has been associated with prior hepatitis B and C virus infection, as well as intravenous drug abuse. The typical findings consist of multiple microaneurysms and distal stenoses in the renal and other mesenteric arterial circulation. The clinical history of fever, elevated sedimentation rate and hepatitis B infection, in the presence of the angiographic findings in the case illustrated, make this the most likely diagnosis.
Polyarteritis nodosa (PAN): PAN is a necrotizing vasculitis of unknown etiology, which has been associated with prior hepatitis B and C virus infection, as well as intravenous drug abuse. The typical findings consist of multiple microaneurysms and distal stenoses in the renal and other mesenteric arterial circulation. The clinical history of fever, elevated sedimentation rate and hepatitis B infection, in the presence of the angiographic findings in the case illustrated, make this the most likely diagnosis.
 Vasculitis of intravenous drug abuse: This entity is also a necrotizing vasculitis, which can have an identical angiographic appearance to PAN. The absence of a history of drug abuse favors an alternative diagnosis, although it must be kept in mind that such a history is often not revealed by the patient.
Vasculitis of intravenous drug abuse: This entity is also a necrotizing vasculitis, which can have an identical angiographic appearance to PAN. The absence of a history of drug abuse favors an alternative diagnosis, although it must be kept in mind that such a history is often not revealed by the patient.
 Vasculitis associated with connective tissue disorders: These include a number of connective tissue disorders (e.g., systemic lupus erythematosus, rheumatoid arthritis, and Sjogren’s syndrome) that have an angiographic appearance similar to the findings in the case illustrated. The diagnosis is based on clinical findings (e.g., arthralgias, cutaneous manifestations) and serologic tests (e.g., antinuclear antibody levels) and is typically known by the time angiography is performed.
Vasculitis associated with connective tissue disorders: These include a number of connective tissue disorders (e.g., systemic lupus erythematosus, rheumatoid arthritis, and Sjogren’s syndrome) that have an angiographic appearance similar to the findings in the case illustrated. The diagnosis is based on clinical findings (e.g., arthralgias, cutaneous manifestations) and serologic tests (e.g., antinuclear antibody levels) and is typically known by the time angiography is performed.
DIAGNOSIS
Polyarteritis nodosa
KEY FACTS
Clinical
 PAN is an immune complex-mediated necrotizing vasculitis affecting multiple organ systems.
PAN is an immune complex-mediated necrotizing vasculitis affecting multiple organ systems.
 PAN can be seen at any age, but the peak incidence is in the fifth to seventh decades. A 2:1 male-to-female predominance has been reported.
PAN can be seen at any age, but the peak incidence is in the fifth to seventh decades. A 2:1 male-to-female predominance has been reported.
 The clinical symptoms reflect the multisystem involvement and include fever, weight loss, weakness, and malaise. Additional symptoms are organ system-specific and vary considerably among patients.
The clinical symptoms reflect the multisystem involvement and include fever, weight loss, weakness, and malaise. Additional symptoms are organ system-specific and vary considerably among patients.
 Laboratory findings include elevated ESR, anemia, and elevated white blood cell count. Rheumatoid factor is present in many patients, and evidence of previous hepatitis B infection is seen in 15%. Elevated circulating immune complexes and hypocomplimentemia strongly suggest the diagnosis.
Laboratory findings include elevated ESR, anemia, and elevated white blood cell count. Rheumatoid factor is present in many patients, and evidence of previous hepatitis B infection is seen in 15%. Elevated circulating immune complexes and hypocomplimentemia strongly suggest the diagnosis.
 Renal involvement, often manifested by rapidly progressive renal failure and hypertension, is present in 75% to 85% of PAN patients. Renal abnormalities can include a necrotizing glomerulonephritis with associated hematuria, vasculitis, and small renal artery aneurysms.
Renal involvement, often manifested by rapidly progressive renal failure and hypertension, is present in 75% to 85% of PAN patients. Renal abnormalities can include a necrotizing glomerulonephritis with associated hematuria, vasculitis, and small renal artery aneurysms.
 Neurologic involvement is common but is usually confined to one or more peripheral or cranial nerves. Less commonly, the brain or spinal cord is involved by infarction or hemorrhage.
Neurologic involvement is common but is usually confined to one or more peripheral or cranial nerves. Less commonly, the brain or spinal cord is involved by infarction or hemorrhage.
 GI involvement is seen in 50% of patients and may involve any organ in the GI system. Bowel ischemia and perforation secondary to necrotizing vasculitis has been reported.
GI involvement is seen in 50% of patients and may involve any organ in the GI system. Bowel ischemia and perforation secondary to necrotizing vasculitis has been reported.
 Cardiac involvement, including congestive heart failure, myocardial infarction, and pericarditis, is seen in 80% of patients.
Cardiac involvement, including congestive heart failure, myocardial infarction, and pericarditis, is seen in 80% of patients.
 Musculoskeletal involvement, including myalgias and a polyarthritis that is clinically similar to rheumatoid arthritis, is also relatively common.
Musculoskeletal involvement, including myalgias and a polyarthritis that is clinically similar to rheumatoid arthritis, is also relatively common.
 Treatment consists of high-dose corticosteroids and cyclophosphamide.
Treatment consists of high-dose corticosteroids and cyclophosphamide.
Radiologic
 Abnormal renal angiograms are seen in 70% to 80% of PAN patients, usually involving small- and medium-sized arteries, often within intrarenal branches. The major findings (many of which are the result of necrosis and inflammation) include multiple microaneurysms (often at bifurcations), segmental narrowing or dilation (due to loss of vasomotor control), and arterial occlusions (due to a necrotizing, inflammatory process).
Abnormal renal angiograms are seen in 70% to 80% of PAN patients, usually involving small- and medium-sized arteries, often within intrarenal branches. The major findings (many of which are the result of necrosis and inflammation) include multiple microaneurysms (often at bifurcations), segmental narrowing or dilation (due to loss of vasomotor control), and arterial occlusions (due to a necrotizing, inflammatory process).
 Involvement of the mesenteric arteries occurs in 65% of PAN patients. The findings are generally similar to those seen in the renal arteries.
Involvement of the mesenteric arteries occurs in 65% of PAN patients. The findings are generally similar to those seen in the renal arteries.
 Appropriate assessment of PAN patients by angiography includes selective renal, celiac, and superior mesenteric injections.
Appropriate assessment of PAN patients by angiography includes selective renal, celiac, and superior mesenteric injections.
SUGGESTED READING
Citron BP, Halpern M, McCarron M, et al. Necrotizing angiitis associated with drug abuse. N Engl J Med 1970;283:1003–1011.
Cupps TR, Fauci AS. Systemic necrotizing vasculitis of the polyarteritis nodosa group. In The Vasculitides. Philadelphia, PA: Saunders, 1981;26–49.
Pipitone N, Versari A, Salvarani C. Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update. Rheumatology 2008;47:403–408.
Stanson AW, Friese JL, Johnson CM, et al. Polyarteritis nodosa: spectrum of angiographic findings. Radiographics 2001;21(1):151–159.
Travers RL, Allison DJ, Brettle RP, Hughes GRV. Polyarteritis nodosa: a clinical angiographic analysis of 17 cases. Semin Arthritis Rheum 1979;8:184–199.
GLENN E.
NEWMAN
AND
CHARLES Y.
KIM
HISTORY
A 27-year-old woman with a painful mass in the posterior aspect of the distal left thigh. The mass was associated with a thrill and bruit.
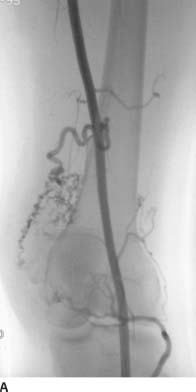
 FIGURE 7-7A Early arterial phase of a right lower extremity angiogram shows a region of multiple prominent tortuous collateral arteries.
FIGURE 7-7A Early arterial phase of a right lower extremity angiogram shows a region of multiple prominent tortuous collateral arteries.
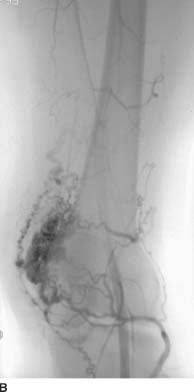
 FIGURE 7-7B Slightly later in the arterial phase, dense opacification of the nidus is seen overlying the lateral condyle of the femur, with several prominent early-filling draining veins.
FIGURE 7-7B Slightly later in the arterial phase, dense opacification of the nidus is seen overlying the lateral condyle of the femur, with several prominent early-filling draining veins.
 Hemangioma: Hemangiomas are benign congenital malformations which are present at birth and typically involute during childhood. At angiography, hemangiomas typically appear as a focal parenchymal blush without large feeding arteries or draining veins. However, in the case shown here, large feeding arteries are present; this diagnosis is incorrect.
Hemangioma: Hemangiomas are benign congenital malformations which are present at birth and typically involute during childhood. At angiography, hemangiomas typically appear as a focal parenchymal blush without large feeding arteries or draining veins. However, in the case shown here, large feeding arteries are present; this diagnosis is incorrect.
 Arteriovenous (AV) fistula: An AV fistula is a direct communication between an artery and a vein without an interposed capillary bed, and can be posttraumatic, iatrogenic, or spontaneous (usually related to atherosclerosis or infection). Although there may be some enlargement of the feeding artery and draining vein, AV fistulas do not recruit collateral vessels. The appearance of multiple tortuous enlarged arteries and veins, as well as a large vascular nidus, is strong evidence against an AV fistula.
Arteriovenous (AV) fistula: An AV fistula is a direct communication between an artery and a vein without an interposed capillary bed, and can be posttraumatic, iatrogenic, or spontaneous (usually related to atherosclerosis or infection). Although there may be some enlargement of the feeding artery and draining vein, AV fistulas do not recruit collateral vessels. The appearance of multiple tortuous enlarged arteries and veins, as well as a large vascular nidus, is strong evidence against an AV fistula.
 Peripheral arteriovenous malformation (AVM): AVMs angiographically are seen to have enlarged tortuous feeding arteries, a nidus, and early filling of enlarged draining veins. All these features are seen on the images in this case, making this the correct diagnosis. Of note, some sources use the terms “AV fistula” and “AVM” interchangeably.
Peripheral arteriovenous malformation (AVM): AVMs angiographically are seen to have enlarged tortuous feeding arteries, a nidus, and early filling of enlarged draining veins. All these features are seen on the images in this case, making this the correct diagnosis. Of note, some sources use the terms “AV fistula” and “AVM” interchangeably.
 Neoplasm: Solid tumors of bone and soft tissues can recruit arterial blood supply, resulting in multiple tortuous feeding arteries. When very large, AV shunting can occur with some degree of early venous filling. However, in the case shown here, the degree and speed of venous return is higher than would be expected in a solid tumor. Furthermore, the density of the nidus is far too prominent to represent a solid tumor during the arterial phase, making this diagnosis incorrect.
Neoplasm: Solid tumors of bone and soft tissues can recruit arterial blood supply, resulting in multiple tortuous feeding arteries. When very large, AV shunting can occur with some degree of early venous filling. However, in the case shown here, the degree and speed of venous return is higher than would be expected in a solid tumor. Furthermore, the density of the nidus is far too prominent to represent a solid tumor during the arterial phase, making this diagnosis incorrect.
DIAGNOSIS
Peripheral arteriovenous malformation
KEY FACTS
Clinical
 Peripheral AVMs are congenital lesions that are caused by abnormal differentiation of the vascular system during embryogenesis, resulting in aberrant vessel angiogenesis.
Peripheral AVMs are congenital lesions that are caused by abnormal differentiation of the vascular system during embryogenesis, resulting in aberrant vessel angiogenesis.
 The nidus of an AVM is the abnormal channel formed by the confluence of the small tortuous vessels, where AV shunting occurs without an intervening capillary bed.
The nidus of an AVM is the abnormal channel formed by the confluence of the small tortuous vessels, where AV shunting occurs without an intervening capillary bed.
 Often, AVMs are not evident until adolescence, when they are stimulated to grow and engorge in response to thrombosis, trauma, infection, or endocrine factors. The AVM usually grows in size with growth of the child.
Often, AVMs are not evident until adolescence, when they are stimulated to grow and engorge in response to thrombosis, trauma, infection, or endocrine factors. The AVM usually grows in size with growth of the child.
 Some AVMs appear as part of a familial genetic disorder such as Rendu-Osler-Weber syndrome, Klippel-Trenaunay, Parkes-Weber, and other syndromes.
Some AVMs appear as part of a familial genetic disorder such as Rendu-Osler-Weber syndrome, Klippel-Trenaunay, Parkes-Weber, and other syndromes.
 AVMs can be stable lesions that require no specific therapy, e.g., solely as a cosmetic deformity. Alternatively, those within extremities can have a clinical presentation of a pulsatile mass or painful lesion. Very large AVMs can produce high-output cardiac failure due to AV shunting.
AVMs can be stable lesions that require no specific therapy, e.g., solely as a cosmetic deformity. Alternatively, those within extremities can have a clinical presentation of a pulsatile mass or painful lesion. Very large AVMs can produce high-output cardiac failure due to AV shunting.
 Some AVMs aggressively expand, necessitating treatment. The primary mechanism of expansion is by enlargement of existing channels and recruitment of new collateral vessels, rather than by cellular proliferation.
Some AVMs aggressively expand, necessitating treatment. The primary mechanism of expansion is by enlargement of existing channels and recruitment of new collateral vessels, rather than by cellular proliferation.
 Low flow vascular malformations include venous malformations and lymphatic malformations. Neither of these have a prominent arterial component.
Low flow vascular malformations include venous malformations and lymphatic malformations. Neither of these have a prominent arterial component.
Radiologic
 Imaging is important for characterizing the size, extent, flow velocity, flow direction, and relationship of AVMs to surrounding structures.
Imaging is important for characterizing the size, extent, flow velocity, flow direction, and relationship of AVMs to surrounding structures.
 MR imaging is excellent for assessing the size, extent, and relationship to surrounding structures. Slow-flow venous malformations have high signal intensity on T2-weighted images, whereas high-flow AVMs are typically seen to have a signal void. Time-resolved MRA is a helpful component of MR imaging of these lesions, because it allows dynamic assessment of the flow velocity and direction.
MR imaging is excellent for assessing the size, extent, and relationship to surrounding structures. Slow-flow venous malformations have high signal intensity on T2-weighted images, whereas high-flow AVMs are typically seen to have a signal void. Time-resolved MRA is a helpful component of MR imaging of these lesions, because it allows dynamic assessment of the flow velocity and direction.
 Sonography can be helpful for assessing superficial lesions, allowing visualization of arterial and venous flow and measurement of velocities.
Sonography can be helpful for assessing superficial lesions, allowing visualization of arterial and venous flow and measurement of velocities.
 Conventional angiography remains the reference standard for diagnosing and assessing treatment options.
Conventional angiography remains the reference standard for diagnosing and assessing treatment options.
 When indicated, AVMs are frequently treated by trans-catheter embolization, alone or in conjunction with surgery. On occasion, AVMs are treated by surgery alone, especially when only one or a few feeding arteries are present.
When indicated, AVMs are frequently treated by trans-catheter embolization, alone or in conjunction with surgery. On occasion, AVMs are treated by surgery alone, especially when only one or a few feeding arteries are present.
 High flow AVMs can be extremely challenging to treat and often require multiple repeat therapies.
High flow AVMs can be extremely challenging to treat and often require multiple repeat therapies.
 Catheter access to the nidus can be accomplished via a direct percutaneous puncture, transarterial route, or transvenous route, depending on the anatomy and location of the lesion.
Catheter access to the nidus can be accomplished via a direct percutaneous puncture, transarterial route, or transvenous route, depending on the anatomy and location of the lesion.
 Transcatheter embolization can be performed using a number of embolic agents. Obliteration of the nidus is considered to be critical for prevention of recurrence, and is ideally performed using a sclerosing agent such as absolute ethanol or glue. Embolization of the feeding arteries and/or draining veins using particles, coils, or other embolic agents and devices may help slow the flow to more effectively treat the nidus, especially for high-flow lesions. However, embolization of the feeding arteries or draining veins without treating the nidus is ineffective because the nidus will recruit collateral vessels, potentially resulting in an even larger lesion.
Transcatheter embolization can be performed using a number of embolic agents. Obliteration of the nidus is considered to be critical for prevention of recurrence, and is ideally performed using a sclerosing agent such as absolute ethanol or glue. Embolization of the feeding arteries and/or draining veins using particles, coils, or other embolic agents and devices may help slow the flow to more effectively treat the nidus, especially for high-flow lesions. However, embolization of the feeding arteries or draining veins without treating the nidus is ineffective because the nidus will recruit collateral vessels, potentially resulting in an even larger lesion.
SUGGESTED READING
Herborn CU, Goyen M, Lauenstein TC, et al. Comprehensive time-resolved MRI of peripheral vascular malformations. AJR Am J Roentgenol 2003;181(3):729–735.
Hyodoh H, Hori M, Akiba H, et al. Peripheral vascular malformations: imaging, treatment approaches, and therapeutic issues. Radiographics 2005;25(Suppl 1):S159–S171.
Lee BB. Critical issues in management of congenital vascular malformation. Ann Vasc Surg 2004;18(3):380–392.
Riles TS, Rosen RJ. Arteriovenous malformations. In DE Strandness Jr, A van Breda (eds), Vascular Diseases: Surgical and Interventional Therapy. New York, NY: Churchill Livingstone, 1994:1109–1137.
Simons ME. Peripheral vascular malformations: diagnosis and percutaneous management. Can Assoc Radiol J 2001;52(4):242–251.
TONY P.
SMITH
AND
CHARLES Y.
KIM
HISTORY
Two patients with hypertension: patient A, shown in Figure 7-8A, is a 67-year-old male with an abdominal bruit; patient B, shown in Figure 7-8B, is a 37-year-old female with hypertension of recent onset. In neither case did the guidewire or catheter enter the renal arteries prior to imaging.
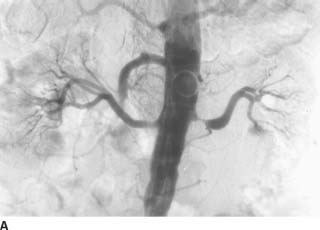
 FIGURE 7-8A Abdominal aortogram shows a smoothly contoured severe stenosis of the proximal left renal artery. Mild stenosis of the proximal right renal artery and abdominal aortic luminal irregularity are also present.
FIGURE 7-8A Abdominal aortogram shows a smoothly contoured severe stenosis of the proximal left renal artery. Mild stenosis of the proximal right renal artery and abdominal aortic luminal irregularity are also present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


