, Yves Etienne2 and Maurice Niddam3
(1)
Faculté de Médecine, Marseille, France
(2)
Unité de Médecine Légale, Hôpital de la Timone, Marseille, France
(3)
Unité SAMU 13, Centre 15, Hôpital de la Timone, Marseille, France
The vascularisation of the amygdala has been much less studied than the vascularisation of the neighbouring hippocampus (which was the subject of excellent research work conducted by HM Duvernoy 1988; HM Duvernoy et al. 2013). However, due to the importance currently related to epilepsy surgery performed as an open procedure or by stereotaxy (E Mempel 1975) and to the need for preoperative stereotaxic investigations in order to detect or identify epileptogenic areas, renewed research has been undertaken on this topic.
9.1 The State of Our Knowledge
Anatomical studies have obviously focused on arterial vascularisation and certain studies have been mentioned in neurosurgical literature (HO Luders 2008).
This study is difficult and misleading because, given the extreme thinness of the arterioles that irrigate the amygdala, we are led to believe that, following a global vascular injection of an anatomical subject and even after a direct injection into the common carotid system (or even better into the internal carotid artery), the vascularisation of the amygdala is poor. Many of the standard contrast products, such as anatomical coloured resins, do not reach the thin arterioles of the amygdala. Thus, in the study performed by R Marinkovic and L Markovic in 1991, the authors referred to the failure of the injections made into the middle cerebral artery: “By injecting the middle cerebral artery, the amygdala body remained unfilled by the mixture”. We encountered the same difficulty! It is therefore necessary to achieve a real assessment of the amount of amygdaloid arteries, not only by performing selective injections in cerebral arteries but also by using very fluid contrasting agents, the most suitable being Indian ink (process used by HM Duvernoy et al. 2013).
Three sources supply the amygdala according to B Goetzen and Sztamska E (1999), a main source and two secondary sources:
The main source is represented by 1–3 branches from the proximal segment of the middle cerebral artery. A single branch corresponds to the most common arrangement. This arteriole, whose diameter is between 200 and 350 μm, penetrates the amygdala, perpendicularly to its surface and then passes toward the rear section. After having progressed over a few mm in the amygdala, it divides into multiple branches.
The second source is one of the secondary sources: It is represented by tiny ramifications (80–100 μm in diameter) of the superficial arterioles (cortical) of the middle cerebral artery.
The third source, which is also secondary, is represented by sparsely branched, thin and straight arterioles, which emerge from the superficial branches (cortical) of the anterior choroidal artery. They penetrate near the uncus and primarily target the basal part of the amygdala. In our experience, we have frequently observed these arterioles and the choroidal source1 is considered by many authors as the main arterial source of the amygdala (G Salamon 1971; G Lazorthes et al. 1976).
We have also observed, on sections injected with ink, very fine branches from the periamygdaloid cortex and adjacent cortical areas, which are orientated perpendicular to the surface of the cortex and which penetrate, at the level of its deep surface, the amygdaloid nuclei. We must finally point out some thin vascular interconnections between the head of the hippocampus and the amygdala in areas where the two formations join up.
Microscopic studies also provided a good appreciation of the arterial vascularisation of the amygdala as they show the very rich and numerous capillaries of this formation.
As for the venous drainage of the amygdala, we know that it essentially takes place into the lower choroidal vein or choroid plexus vein which emerges from the temporal horn of the lateral ventricle and targets the basal vein.
It should also be recalled that the amygdala is largely in contact with the subarachnoid spaces (on the one the hand, at the uncal recess of the temporal horn of the lateral ventricle and, on the other hand, at the level of the lateral part of the transverse fissure) and therefore with the cerebrospinal fluid, whose role of transporting neurotransmitters is not the least, in addition to its nutritional and mechanical functions. The choroid plexus of the lateral ventricle only appears beyond the hippocampal head but immediately provides thin vascular branches for the cortical and baso-medial nuclei of the amygdala (Fig. 9.1).
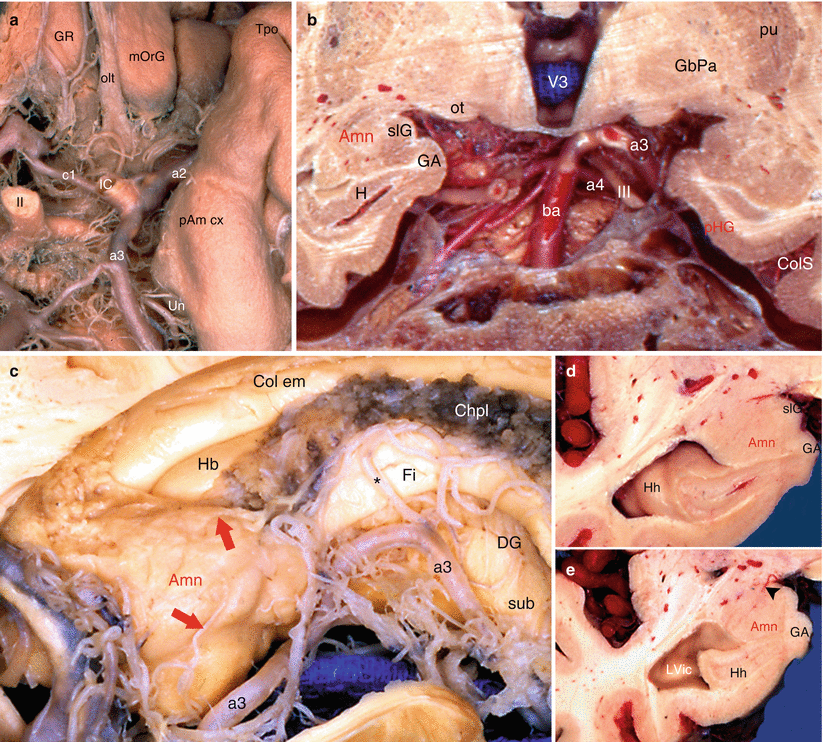

Fig. 9.1




Arterial vascularisation of the amygdaloid nuclear complex. (a) inferior view of the arteries edging with the left amygdalo-hippocampal area; (b) coronal section of the brain in front of the basilar artery (arteries injected by a red-coloured resin); (c) dorsal view of the amygdalo-hippocampal area; (d, e) coronal sections targeting the amygdala to show the arteries of the neighbourhood. Amn amygdaloid nuclear complex, ba basilar artery, Chpl choroid plexus, Col em collateral eminence, ColS collateral sulcus, DG dentate gyrus, Fi fimbria, GA gyrus ambiens, GbPa globus pallidus, GR gyrus rectus, H hippocampus, Hb hippocampus body, Hh hippocampus head, IC internal carotid, LVic lateral ventricle, inferior cornu, mOrG medial orbital gyrus, olt olfactory tract, ot optic tract, pAm cx periamygdaloid cortex, pu putamen, slG semilunaris gyrus, sub subiculum, Tpo temporal pole, Un uncus, V3 third ventricle, a2 middle cerebral artery, a3 posterior cerebral artery, a4 superior cerebellar artery, c1 anterior communicating artery, II optic nerve, III oculomotor nerve, red arrows they show two arterial rami ending in the superior face of the amygdala, black arrowhead showing a ramus from a3, black asterisk posterolateral choroidal ramus
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree