Overgrowth syndromes, particularly within the PIK3CA-related overgrowth syndrome (PROS) spectrum, are commonly associated with venous anomalies. The anomalies include spongiform venous malformations and persistent embryonic veins, such as the lateral marginal vein (of Servelle). The anomalous veins pose a significant risk of thromboembolic disease and should be occluded, preferably earlier in life. A thorough understanding of the conditions, anatomy, and interdisciplinary treatment of these complex anomalies is essential for optimal management. This review explores the clinical and imaging diagnosis of overgrowth syndromes and techniques for assessing and treating associated venous anomalies, particularly the endovenous closure of anomalous veins.
Introduction
Overgrowth syndromes encompass a range of disorders characterized by abnormal tissue proliferation in multiple body regions. They are primarily triggered by genetic mutations that disrupt essential cellular functions of metabolism, survival, proliferation, and growth. Disorders contained within the PIK3CA-Related Overgrowth Spectrum (PROS) result from mosaic somatic mutations in the PIK3CA gene. These mutations initiate a continuous signaling cycle that impedes apoptosis and encourages cell proliferation and migration, leading to excessive tissue expansion and angiogenesis – both key traits of these syndromes. ,
Certain PROS phenotypes are highly associated with vascular anomalies, including disorders such as Klippel-Trenaunay Syndrome (KTS), CLOVES (congenital lipomatous overgrowth with its associated vascular malformations, epidermal nevi, spinal and skeletal abnormalities, and/or scoliosis), FAVA (fibroadipose vascular anomaly), CLAPO (capillary malformation, lymphatic malformation), and MCAP (megalencephaly-capillary syndrome). , These disorders exhibit a range of overlapping phenotypic features and symptoms, with severity often corresponding to the degree of tissue overgrowth and vascular anomalies.
Klippel-Trenaunay Syndrome (KTS) and CLOVES syndrome, in particular, are distinguished by their high prevalence of complex and multifaceted vascular anomalies. These span from superficial skin-related vascular malformations to potentially life-threatening venous system anomalies that put individuals at a heightened risk of thromboembolic events. , For the interventional radiologist, knowledge of the clinical presentation, imaging findings, and recognition of these venous anomalies is crucial. This knowledge allows for planning appropriate multidisciplinary treatment strategies that can mitigate potential harm and improve the quality of life for those affected.
This article examines the clinical and imaging features of venous anomalies associated with PIK3CA-Related Overgrowth Spectrum (PROS) disorders, focusing on Klippel-Trenaunay Syndrome (KTS) and CLOVES syndrome. It also discusses image-guided treatment strategies for these conditions.
Overview of KTS and CLOVES syndrome
Klippel-Trenaunay Syndrome (KTS) and CLOVES syndrome are PROS phenotypes characterized by marked tissue overgrowth and vascular anomalies. The vascular anomalies associated with these syndromes can cause considerable morbidity and necessitate comprehensive diagnostic and management strategies. , , ,
Klippel-Trenaunay Syndrome (KTS) is characterized by capillary malformations, slow-flow vascular malformations (lymphatic and/or venous), and localized tissue overgrowth predominantly affecting a single lower limb. Diagnostic features of KTS typically become evident during a comprehensive physical examination, which usually reveals distinctive signs of capillary, lymphatic, and venous malformations. These signs include skin discoloration, lymphatic vesicles, varicosities affecting a lower extremity, and asymmetrical limb overgrowth. The limb overgrowth is predominantly due to microcystic lymphatic malformation and associated fatty hypertrophy and is therefore largely extrafascial.
CLOVES syndrome is characterized by congenital lipomatous overgrowth, visible as palpable soft masses primarily localized to the trunk. It also features a mix of slow- and fast-flow vascular malformations, often manifesting as varicosities or palpable soft tissue masses involving the limbs and trunk. , Patients typically exhibit epidermal nevi, skeletal truncal anomalies such as scoliosis, and/or asymmetric limb overgrowth (also characteristic of KTS). Given its rarity and overlapping symptomatology, a diagnosis of CLOVES syndrome can sometimes be missed or mistakenly attributed to KTS.
Venous anomalies in KTS and CLOVES syndrome
Both CLOVES and KTS are associated with multiple types of venous anomalies, particularly in the pelvic region. This may manifest as rectal bleeding if there is anorectal involvement, and hematuria if there is genitourinary disease.
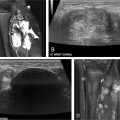
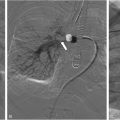
A characteristic venous anomaly frequently observed in KTS and CLOVES patients is the persistence of embryonic veins, commonly affecting the lower extremities or trunk ( Fig. 1 ). These anomalous veins often display ectasia, incompetence, and an increased likelihood of thromboembolism, posing a potential risk for life-threatening pulmonary embolism (PE). One well-known persistent embryonic vein is the lateral marginal vein (LMV), a vessel that normally regresses during development. , In KTS and CLOVES syndrome, however, this regression process is disrupted, leading to an abnormal and enlarged superficial vein.

The persistent LMV typically originates at the dorsal venous arch of the foot and comprises multiple popliteal and infrapopliteal tributaries. It follows an ascending, tortuous course within the subcutaneous tissue along the lateral aspect of the lower limb, usually spanning the lower leg, knee, and thigh. Its drainage pattern into the internal iliac vein or saphenofemoral venous circulation is variable. The LMV generates numerous perforating veins that establish connections between the superficial and deep venous systems, forming a complex network of collateral pathways. These connections may extend beyond the anticipated orthotopic tributaries, occasionally involving ectopic ones, as well as gluteal and pelvic-retroperitoneal venous channels. , , Oduber et al. classified the persistent lateral marginal vein (LMV) into 4 types based on its drainage pattern. However, a recent MRI study of 51 patients with LMV found the Oduber classification to be unreliable due to the vein’s high anatomical heterogeneity. This study suggests an updated system reflecting current imaging technology and varied disease presentations.
Both KTS and CLOVES can involve orthotopic venous anomalies within the deep venous system that can extend into the central trunk. , The dilation of orthotopic veins in these patients serves as a manifestation of the underlying vascular dysmorphogenesis responsible for the persistence of embryonic veins. The persistent LMV is frequently associated with significant valvular insufficiency, further exacerbating the prevailing venous hypertension observed in these patients. ,
Approximately 13% of cases with persistent LMV exhibit deep venous aplasia or hypoplasia. In KTS patients undergoing surgical treatment, additional deep venous anomalies have been seen to involve the popliteal vein (51%), femoral vein (16%), both femoral and popliteal veins (29%), iliac vein (3.3%), and inferior vena cava (0.7%).
Another persistent embryonic vein that may contribute to venous insufficiency in KTS and CLOVES syndrome is the persistent sciatic vein (PSV). As a major venous pathway, the PSV is primarily responsible for draining the posterior compartment of the lower limb. It follows the course of the sciatic nerve, ascending through the posterior thigh while receiving tributaries from that area. Like the LMV, the PSV drainage pattern can vary.
The PSV drainage pattern can be described in 3 distinct anatomical variations. The first is considered “complete” when the PSV extends along the entire length of the thigh and buttock. It originates either from the popliteal vein or nearby tributaries, traverses the sciatic notch, and ultimately terminates in the internal iliac venous system. The second variation, known as “upper” PSV, is limited to the buttock and upper thigh. It arises from small tributaries or embryonic subcutaneous veins, traverses the sciatic notch, and terminates in the pelvis. Lastly, the “lower” PSV is confined to the distal and middle thigh, terminating either in the deep femoral venous system or an embryonic subcutaneous venous network.
In KTS and CLOVES syndrome, venous anomalies often extend beyond the lower extremities and can be associated with ectasia of the iliac veins and IVC. CLOVES patients may also exhibit involvement of the upper extremities and ectasia of the thoracic orthotopic veins, particularly the subclavian and brachiocephalic veins. , Additionally, CLOVES syndrome can be associated with an ectatic, anomalous vein in the lateral thoracic wall ( Fig. 2 ). Other anomalies, such as azygos continuation of the IVC or persistent left SVC, elevate the risk of thrombosis and PE.

Before any endovascular intervention in patients with these syndromes, a thorough assessment of vascular anatomy is vital given the complexity of their vascular anomalies. Meticulous utilization of noninvasive imaging and venography techniques is essential for gaining a comprehensive understanding of anatomical variations and ensuring a safe and effective treatment approach. , ,
Noninvasive imaging findings in PROS (KTS and CLOVES)
In the evaluation of KTS and CLOVES syndrome, magnetic resonance imaging (MRI) is an indispensable tool for assessing the extent of soft tissue overgrowth and vascular malformations. , , , It provides superior soft tissue contrast and comprehensive anatomical characterization. MRI can reveal soft tissue and bone hypertrophy, as well as venous malformations, which typically appear as enlarged tubular or lobulated areas of high signal intensity on T2-weighted sequences. Thrombi and phleboliths may be identified as foci of low T1/low T2 signal intensity. Lipomatous overgrowth can also be detected, usually appearing as areas of high signal intensity on T1-weighted images, with signal loss upon fat saturation.
Magnetic Resonance Angiography provides detailed images of the vascular system and can be particularly useful for imaging dilated vessels in the deep venous system. On MR Venography (MRV), anomalous veins appear as dilated, twisted, irregular structures with slow flow or contrast retention. Additionally, MRV can detect the absence or hypoplasia of deep veins – a critical observation given the associated elevated risk of deep vein thrombosis. However, it’s important to note that MRV may underestimate the condition of the deep venous system due to flow steal by larger anomalous veins. It should not be considered an alternative to basic MRI sequences. In addition, due to patient positioning, the presence of a sciatic vein and its size are likely underestimated on MRI. As the marginal vein is readily identifiable on basic MRI sequences, if conventional venography and possible endovenous closure are planned, MRV is unnecessary in most cases. Any initial MRI of the lower extremity should include imaging of the pelvis to identify venous malformations and the abdomen to detect any ectasia of the portal venous system. An ectatic portal venous system, particularly a dilated inferior mesenteric vein, increases the risk of portal vein thrombosis.
Ultrasound (US) is a readily accessible, noninvasive method that delivers immediate dynamic information without ionizing radiation. It’s particularly adept at identifying vascular malformations and assessing their flow characteristics using Doppler US. US can detect phlebectasia, venous malformations, and persistent embryonic veins such as the lateral marginal and sciatic veins. These anomalies typically appear as enlarged, tubular hypoechoic structures with slow flow, fluid-fluid levels, thrombi, and/or phleboliths. US can also help discern deep venous anomalies (eg, absence or hypoplasia of deep veins) and lipomatous overgrowth, which appears as hyperechoic regions potentially displacing or compressing adjacent veins.
While computed tomography (CT) scans have their uses in certain cases (such as imaging bones, abdominal structures, and acute pulmonary embolism), their application in this context is limited.
Before undertaking interventions on abnormal veins, venography is commonly performed at our institution to assist in the planning and execution of the procedures.
Venography
Venography plays a vital role in the evaluation of complex venous anomalies in patients with KTS and CLOVES Syndrome. It provides crucial information for treatment planning and monitoring treatment effectiveness. In our institution, we employ a comprehensive venography approach involving a minimum of 2 access sites for evaluating the affected lower extremity. Typically, the deep venous system is accessed at the ankle level using a 20- or 22-gauge angiocatheter or by positioning the inner dilator of a micropuncture sheath into either the posterior tibial vein (PTV) or anterior tibial vein (ATV). Digital subtraction venography is then performed to visualize the entire lower extremity, pelvis, and abdomen. If the filling of superficial veins hampers clear visualization of the deep venous system, diversion venography is conducted. This involves applying 1 or more tourniquets near the perforators of dilated superficial veins, with a focus on the connecting branches between the ectatic superficial veins and the deep veins.
We employ a similar approach for accessing the superficial system in the lower extremity, with the lateral marginal vein accessed in the dorsum of the foot. In many cases, selective catheter-directed venography is carried out using access points in the marginal and sciatic veins to examine the drainage pattern into the iliac veins and the inferior vena cava (IVC). We thoroughly assess the patency of the deep system and the connecting branches between the superficial and deep systems. In situations where multiple overlapping veins hinder a clear distinction between orthotopic and embryonic veins, venography in lateral and oblique projections is performed to enhance vessel resolution.
In CLOVES syndrome, the upper extremities are similarly investigated with access into the deep veins in both upper extremities and catheterization of the anomalous vein in the lateral thoracic wall.
Venous closure techniques
Endovascular interventions are considered a safe and effective treatment option for KTS and CLOVES Syndrome patients who have not responded to conservative management. The choice of technique for venous closure depends on the anatomical location of the vein being treated. Deep (intrafascial) veins are predominantly closed using coil embolization, whereas superficial (extrafascial) veins are primarily addressed through endovenous laser treatment (EVLT). In instances where extremely large superficial veins do not respond to EVLT, an alternative approach of ligation and/or phlebectomy is chosen. Similarly, for large veins located around the ankle and foot, the preferred course of action is typically ligation, phlebectomy, or sclerotherapy. These veins are often either too short or tortuous for EVLT. There are newer alternatives to EVLT, including Venaseal, which may be suitable for a select population.
Embolization
In our institution, we employ coil embolization for endovenous closure of anomalous veins in specific scenarios. These include phlebectasia of intrafascial veins, situations where nerve proximity makes endovenous laser therapy (EVLT) impractical (such as the sciatic vein), and instances involving the upper end of marginal veins. We initiate coil embolization as centrally as possible. If there is ectasia of the internal iliac vein, the coils are positioned just below the confluence with the external iliac vein and extend caudally. Next, the sciatic vein is coiled down to the confluence with the popliteal vein. Similarly, the intrafascial/pelvic terminal portion of the marginal vein and associated draining veins (such as the gluteal vein) are coil embolized to the level of the fascia.
In cases where exceptionally large veins are involved, we slightly modify the approach. We initially deploy coils in small side branches, serving as anchors to create a framework for placing additional coils. This strategic approach effectively minimizes the risk of coil migration. If anchoring of coils proves technically unfeasible, an inferior vena cava (IVC) filter can be placed in the ectatic veins to provide the necessary scaffold. The anomalous veins are very pliable, so the coils should be oversized, typically to twice the measured size of the vein. The coils should be packed tightly at the superior end to occlude the vein and prevent nontarget embolization, but can be coiled more loosely below, particularly if using fibered coils. In instances where the grossly ectatic veins exceed the diameter of the largest coils we have de-used de-cored Bentson guidewires (Cook Medical, Bloomington, IN).
We avoid the use of coils, plugs, and glue in certain areas, such as near joints and in superficial veins where they may be palpable or erode through the skin.
Endovenous laser treatment
Endovenous laser treatment (EVLT) is primarily employed to treat phlebectasia in extrafascial locations, most commonly in the lateral marginal vein and saphenous vein. To ensure thorough closure of the vein and minimize the risk of thromboembolism cranial to the ablated vein, coils are strategically placed in the intrafascial cranial portion of the vein and associated draining veins.
Tumescent saline is carefully injected around the vein to create compression and serve as a thermal buffer to protect surrounding structures. Given the limitations of dosage in children and the fact that these procedures are performed under general anesthesia, we do not add local anesthetic to our tumescent. The goal is to administer 100 Joules of energy per centimeter, a dosage commonly proven effective for vein ablation while simultaneously minimizing the likelihood of potential complications. In contrast to the greater saphenous vein, where there is a continuous perivascular sheath in 85% of cases, no sheath is present around the lateral marginal vein. This leads to rapid dispersion of tumescent, necessitating staged ablation of the vein, typically in 5-10 cm segments.
The efficacy of EVLT in treating extrafascial venous anomalies in KTS and CLOVES syndrome can be maximized through a combination of coil placement in the intramuscular tributaries, tumescent saline injection, precise energy delivery, and the utilization of a highly advanced laser system. Collectively, these elements work together to enhance the safety and efficacy of the procedure. , Following EVLT, if a catheter can be gently advanced through the ablated vein, we will often sclerose the vein by lacing it with a small volume of STS as the catheter is gently withdrawn. This not only helps to reduce the risk of recanalization of the treated vein but can also result in the sclerosis of the tributaries.
Percutaneous vein ligation
Percutaneous vein ligation is the preferred treatment for certain venous anomalies when coil embolization or laser therapy are not feasible, such as markedly dilated superficial veins or large communicating branches. It involves tying off the anomalous vein to redirect blood flow.
A Keith needle is threaded with a 2-0 silk or Prolene suture and inserted through a small incision adjacent to the vein. Using ultrasound guidance, the needle is passed beneath the vein and brought out through another small incision on the opposite side. It is then redirected back through the exit incision above the vein (still under ultrasound guidance) and brought out through the original entry site. The 2 ends of the suture are tied together to form a loop, effectively ligating the vein. The ligation knot is tucked under the skin for smoother healing and reduced infection risk. Finally, the incisions on both sides of the vein are sealed with Steri-Strips.
Phlebectomy
Ultrasound-guided phlebectomy is utilized to treat superficial veins or complex venous segments that are unsuitable for laser therapy, coil embolization, or percutaneous ligation.
Using sonographic guidance, a small incision is made above the abnormal vein. Small curved forceps are used to carefully dissect the surrounding tissue until the vein is free. The vein is then grasped with the forceps, and gradual, continuous traction is applied to divide and extract it.
Hemostasis is typically achieved through manual compression. In cases where manual compression is ineffective, the transected vein or its feeding branches can be ligated using 2-0 or 3-0 silk sutures. Finally, the incision sites are closed using either sutures or Steri-Strips.
Sclerotherapy
Sclerotherapy is used for veins that are too tortuous for EVLT and for intrafascial veins that do not have evidence of rapid egress into deep veins. To prepare the foam sclerosant, a 3% solution of sodium tetradecyl sulfate (STS) is mixed with air and ethiodized oil in a 2:1:1 mixture. Under ultrasound and fluoroscopic guidance, the STS foam is carefully administered into the vein, ensuring accurate placement and monitoring for unintended leakage. In longer segments of vein, a catheter can be advanced and gradually retracted while continuously injecting the sclerosant. This evenly disperses the foam throughout the vein’s entire length. In smaller, more tortuous veins, the sclerosant can be injected directly through a hypodermic or Echotip needle following initial venography. Foam maximizes contact between the sclerosant and the vein walls, promoting optimal closure. During sclerotherapy, the flow of saline through the deep venous access can be increased to flush and dilute any egress of sclerosant into deep veins.
VenaSeal
VenaSeal (Medtronic, Minneapolis, MN) is a nonthermal, nontumescent catheter system that seals refluxing superficial veins using a small amount of cyanoacrylate-based adhesive. The adhesive polymerizes upon contact with blood, closing the vein, which is eventually absorbed by the body.
Under ultrasound guidance, a small puncture is made above the targeted vein, and a 7 French introducer sheath is inserted. A single-use cartridge of cyanoacrylate-based adhesive is loaded into the VenaSeal closure system’s dispensing gun. The catheter is then advanced through the sheath to the proximal part of the vein. As the catheter is slowly retracted, the adhesive is gradually infused into the vein. Manual compression is promptly applied after the adhesive is delivered to ensure thorough closure of the vein. This process is repeated along the entire length of the vein. After the procedure, the access site is bandaged.
By omitting the use of tumescent anesthesia, the procedure duration is shortened, and the discomfort associated with multiple needle sticks is avoided, promoting a faster recovery.
However, not all patients are suitable candidates for VenaSeal. Individuals with allergies to n-butyl cyanoacrylate or those with veins that are either too small or too tortuous to safely accommodate the catheter may not be suitable for this technique.
Conclusion
Overgrowth syndromes are complex genetic disorders characterized by excessive tissue growth, and are often accompanied by vascular anomalies that can significantly increase the risk of thrombotic events and other detrimental complications. Among these syndromes, Klippel-Trenaunay syndrome (KTS) and CLOVES syndrome are specific subsets that manifest with overgrowth and complex venous anomalies often including the presence of persistent embryonic veins. A range of minimally invasive interventions are safe and effective therapeutic options for closure of these anomalous veins. These include coil embolization, endovenous laser treatment (EVLT), percutaneous venous ligation, phlebectomy, sclerotherapy, and VenaSeal. Selection of the most appropriate treatment modalities requires a thorough clinical and radiologic evaluation and an individualized interdisciplinary therapeutic approach.
Acknowledgments
None.
Conflict of Interest: None.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree