Chronic abdominal visceral pain management often requires multidisciplinary collaboration. Image-guided visceral nerve interventions may be critical in the management of visceral pain refractory to medical treatments. Abdominal and pelvic pain is mediated by specific nerves involving specific ganglia. The well-defined location of these ganglia provides important targets for percutaneous image-guided interventions to alleviate chronic abdominal or pelvic pain. In this review, we provide an in-depth discussion of the anatomy, indications, evidence, and technical and clinical considerations and complications for celiac plexus, superior hypogastric, inferior hypogastric, and ganglion impar block and neurolysis.
Introduction
Introduction to visceral nerve intervention
The treatment of abdominal pain from chronic diseases remains a challenge, often requiring a multidisciplinary approach for effective management. Although various pharmaceutical options are available to clinicians to manage chronic pain, such options may be morbid to patients, cause side-effects, and may be inefficient at managing certain abdominopelvic chronic pain-generating etiologies, particularly in patients with cancer or chronic inflammatory conditions.
Enhancements of imaging modalities and their subsequent adoption for guiding minimally invasive interventions has allowed physicians to tackle intractable chronic pain, obviating the sole reliance on systemic pharmaceutical agents such as opioids which may be associated with dependence, nausea, vomiting, constipation, and sedation. Interventions such as celiac plexus, superior hypogastric, and ganglion impar block and neurolysis have been increasingly utilized to alleviate or palliate abdominopelvic chronic pain of variable locoregional etiologies. As these interventions become more readily deployed, it is crucial to obtain a proper understanding of their indications, techniques, advantages, disadvantages, complications, and contraindications. Therefore, this review aims to provide a comprehensive overview of these visceral nerve plexus blocks and neurolysis techniques and their applications, emphasizing the importance of precise anatomical knowledge and technical proficiency for successful outcomes.
Specific procedures
Celiac plexus block and neurolysis
Image-guided celiac plexus block and neurolysis (CPBN) is a minimally invasive procedure for the treatment of chronic abdominal pain secondary to abdominal visceral malignancy or chronic inflammation. Celiac plexus block and neurolysis are 2 distinct treatments performed with similar techniques. Celiac plexus block (CPB) is a temporary disruption of pain transmission via injection of corticosteroids or long-acting anesthetics, while neurolysis (CPN) describes permanent destruction of the plexus by injection of ethanol or phenol. CPB is also considered for the treatment of chronic inflammation, including chronic pancreatitis. The successful relief of the pain of pancreatic cancer and other abdominal malignancies can be expected in 85% and 73% of patients, respectively. Most commonly, patients present with epigastric pain that radiate to the back secondary to pancreatic cancer. The mechanism of the pain is multifactorial, involving blockage in the pancreatic duct, increased parenchymal pressure, and superimposed peritumoral edema. Neuropathic pain associated with infiltration of nerves by the cancer occurs in 70%-90% of cases.
Anatomy
The celiac plexus, arising at the level of T12 through L2, is located bilaterally in the periaortic fat pads of the anterolateral surface of the aorta at the level of the diaphragm hiatus and the celiac trunk. It consists of visceral afferent, as well as sympathetic, and parasympathetic efferent fibers. As the largest visceral plexus, it serves to transmit visceral pain of the pancreas, diaphragm, stomach, liver, spleen, small bowel, transverse colon, suprarenal glands, kidneys, abdominal aorta, and the mesentery. Pain from pancreatic malignancy is believed to be most commonly due to neural invasion and the stimulation of visceral afferent neural fibers which travel from the celiac plexus through the splanchnic nerves. , Further, the plexus also transmits sympathetic efferent fibers derived from the greater, greatest, and least splanchnic nerves that originate from the T5 to T12 sympathetic ganglia, as well as parasympathetic supply derived from the posterior trunk of the vagus nerve, which accounts for the frequently-reported postprocedural hypotension and diarrhea.
Technique
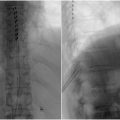
Historically, percutaneous and surgical neurolysis have been the accepted approaches for treatment. Image guidance is chosen based on provider preference and resource availability and includes fluoroscopy, computed tomography (CT), CT fluoroscopy, cone-beam CT, ultrasound (US), or magnetic resonance imaging (MRI). For interventional radiologists, CT-guided percutaneous CPBN has been the mainstay approach. While percutaneous CPBN is typically performed from a posterior approach, the anterior approach is also effective and may be used for cases where the patient cannot tolerate the prone position or if there is an ileostomy or colostomy ( Fig. 1 ). The anterior approach poses may pose more risk to the patient as it requires the needle to traverse multiple visceral organs, although this is not well-established.

The posterior approach can be subdivided into antecrural, retrocrural, transintervertebral disc, and transaortic techniques, and may be chosen based on patient anatomy and position ( Figs. 1 and ( 2 ). The transaortic approach allows the proceduralist to reach the celiac plexus bilaterally with a single puncture, but is contraindicated for patients with existing abdominal aortic aneurysm. The retrocrural approach may be considered if there is significant tumor infiltration around the origin of the celiac and super mesenteric arteries which may impede spread of the injectate; this technique is also known as splanchnic nerve block/neurolysis as the target becomes the greatest, lesser, and least splanchnic nerves which are derived from the T5 to T12 sympathetic ganglia.

Preprocedurally, patients are seen in the clinic and assessed for pain levels and provided information regarding the procedure, expected outcomes, and potential complications of the procedure. It should be noted to patients that CPN may not result in complete pain relief and may result in transient hypotension and diarrhea postprocedurally. The procedure is performed with moderate sedation, though comorbidities and other factors may escalate to monitored anesthesia care.
The procedure is typically performed with a 20 to 24G bevel-tipped needle. The proceduralist begins with an initial CT scan to determine the safest needle trajectory. Then, under local anesthesia, the needle is advanced incrementally with repeated CT image confirmation. Once the needle lands at the desired location, aspiration is performed to confirm location and a selected agent is injected into the retroperitoneal space. If a block is desired, the next step entails injection of an anesthetic such as bupivacaine and steroid (e.g., betamethasone). If treatment is desired, neurolysis is performed with either a mixture of greater than 50% ethanol to induce permanent nerve damage or 3%-20% phenol. , , The key advantage of phenol is its intrinsic anesthetic effect leading to less pain during injection; however, phenol may be more difficult to use/mix as it is viscous compared to ethanol. There are a number of reports on various concentrations of bupivacaine or ropivacaine, varying from 0.25% to 0.75%, in the injectate to minimize discomfort for the patient. A single cocktail of ethanol, bupivacaine, and contrast in a 6:3:1 ratio has also been reported. Steroids, such as triamcinolone or betamethasone, have also been used in the mixture for patient comfort.
Advantages/disadvantages
Visceral neurolysis and block have been shown to effective in reducing chronic pain in many studies. In a randomized control trial (n = 100) where patients were randomly assigned to receive either a celiac neurolysis block or systemic analgesic therapy alone with a sham injection, it was concluded that CPBN improves pain relief in patients with pancreatic cancer compared optimized systemic analgesic therapy alone (14% vs 40%; P = 0.005).
More recently, endoscopic ultrasonography (EUS)-guided celiac plexus neurolysis (EUS-CPN) has been more widely accepted, given several advantages over radiologic and surgical techniques, including enhanced needle precision, the ability to inject the neurolytic agent into a larger area to offer superior analgesia, and the ability to perform alongside tumor biopsy and staging. However, the disadvantage of this approach is significantly more procedure time. While MRI imaging affords superior imaging quality, CT-guided CPBN is more accessible and typically requires less procedural time. Fluoroscopy can be also utilized but provides inferior 3D visualization compared to CT imaging. CT guidance also provides excellent visualization of the injected agent when diluted with contrast. Despite these practical considerations, there is currently a dearth of literature addressing head-to-head comparisons between CPN techniques. As a result, endoscopic, percutaneous, and surgical approaches to CPN are considered equally effective. Proceduralists may select a technique based on the technology availability, experience, and patient needs.
Complications and contraindications
Celiac plexus block and neurolysis is generally considered to be a low-risk procedure; most adverse effects are transient and serious complications occur in less than 2% of cases. Relative contraindication for this procedure includes severe uncorrectable coagulopathy, thrombocytopenia, abdominal aortic aneurysm, untraditional celiac trunk origin, and inability to visualize local anatomy due to cancer mass. For each approach, complications are related to the structures that the needle traverses. For example, the anterior approach may result in gastric perforation, chemical peritonitis, pancreatic fistula, and subcapsular liver hematoma. Typically, the shortest distance from the surface of the skin to the target site is selected to minimize these complications. Notable complications of the retrocrural approach include vascular injury to the anterior spinal cord resulting in paraplegia as well as retrocrural hematoma. The prone position poses a risk of pneumothorax, but care can be taken to coordinate needle advancement with breathing as well minimized with CT gantry angulation.
Superior hypogastric plexus block and neurolysis
Superior hypogastric plexus (SHP) block and neurolysis is used in interventional radiology for acute or chronic pelvic pain. Particularly, patients with pelvic malignancies experiencing recalcitrant pain benefit the most from this procedure as they are commonly tolerant to oral, and sometimes even intravenous, analgesics such as opioids. Other notable indications for SHP block and neurolysis include chronic intractable pelvic pain generated by endometriosis, proctalgia, ilioinguinal neuralgia, or postherniorrhaphy pain. In addition to pain alleviation, SHP block may be used for diagnosing pain origin or regional anesthetic purposes prior to pelvic procedures, such as uterine artery embolization.
Anatomy
The superior hypogastric plexus is located retroperitoneally at the L5-S1 vertebral level, anterior to the bifurcation of the aorta and the common iliac vessels. The SHP primarily receives afferent sympathetic input from the lumbar splanchnic nerves, particularly those arising at the L1 and L2 vertebral levels and provides efferent innervation to various pelvic organs including the bladder, urethra, uterus, prostate, and the rectum. The nerve fibers arising from the SHP descend into the pelvis as the hypogastric nerves to form the inferior hypogastric plexus, making it an effective target for anesthetic interventions aimed at alleviating acute or chronic pelvic pain of various etiologies, most commonly being preprocedure for uterine fibroid embolization. , , ,
Technique
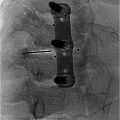
Different imaging modalities may be used to guide interventionalists when performing a SHP block or neurolysis, which requires precise needle placement to minimize surrounding visceral involvement. The most common technique used by IR is using an anterior approach under fluoroscopic guidance. The image intensifier is setup in a craniocaudal angle to ensure enface visualization of the L5 vertebrae and the lower end place is targeted using a Chiba needle. Traditionally, fluoroscopic or CT-guidance is used to initially plan and map the needle trajectory and approach, ensuring proper identification of critical surrounding visceral organs ( Fig. 3 ). Thereafter, a needle is advanced targeting the anterior aspect of the L5 vertebral body. A posterior transintervertebral disc approach may also be considered. Once the needle is safely advanced into position, contrast is injected to confirm correct needle placement before administering the injectate, which is composed of a local anesthetic, neurolytic blocking agents, or a combination of both. Commonly used injectates include steroids such as dexamethasone, ethanol, bupivacaine (alone or prior to alcohol injection), and phenol. , , Such agents have been shown to have short to long term lasting pain-relieving effects lasting up to 12 months. Alternatively, more recent advancement in ultrasound technology has enabled physicians to use this modality more reliably for SHP block and neurolysis guidance. Mishra et al. highlighted the utility and reliability of ultrasound for SHP block or neurolysis using an anterior abdominal approach. The increasing adoption of ultrasonography to administer SHP block and neurolysis in the hands of experienced interventionalists reduces patient exposure to harmful radiation when using fluoroscopic or CT guidance.