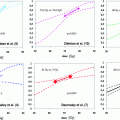
Fig. 1
Axial dose distribution of prostate bed 7-field IMRT a with and b without an endorectal balloon
In order to be well protected against too small clinical target volume, it is useful to consider the anatomy-based target volume definition of the Toronto group (Wiltshire et al. 2007) and in addition to use the RTOG consensus definition (Michalski et al. 2010a). The RTOG consensus decreased the inferior border of the CTV from 8 mm in the guideline from Toronto to at least 8–12 mm below the vesicourethral anastomosis. The superior boundary was defined by the superior surgical clips if present or 5 mm above the inferior border of the vas deferens by the Toronto consensus. The RTOG guideline modified that border so that the CTV usually had not to extend more than 3–4 cm above the level of the symphysis in general. Seminal vesicles remnants have to be included in the clinical target volume according to the RTOG consensus. The posterior caudal boundary is the anterior border of the rectal wall and the levator ani and may be somewhat concave around the anterior–lateral aspect of the rectum. The posterior cranial boundary is the mesorectal fascia. The lateral caudal boundaries are the medial borders of the levator ani and obturator internus muscles. The lateral cranial borders are defined by the sacrorectogenitopubic fascia. The anterior borders are the posterior edges of the pubic bones inferiorly. Above the superior aspect of the pubic bone, the CTV retracts posteriorly and encompasses the 1–2 cm of the posterior bladder wall at the minimum. With that definition, more than 1,000 patients were treated within the RTOG-0534 trial to the prostate bed alone or to the prostate bed in the second series after pelvic radiotherapy including pelvic nodal stations.
In the case of gross residual or recurrent disease, the macroscopic tumor has to be included with a margin of 1 cm according to the Toronto guidelines. In addition, surgical clips outside the above boundaries should be included excluding high lymphadenectomy vessel clips.
4 Conclusion
Postoperative adjuvant or salvage radiotherapy can offer a substantial chance of long-term freedom from PSA progression. The risk of severe late effects of this treatment modality is usually low, as found in the randomized trials on early postoperative radiotherapy using 3D techniques. Modern IMRT radiotherapy techniques can reduce the rectum and bladder exposure further. Care has to be taken to cover the posterior and superior aspects of the prostate bed.
References
Ishiyama H, The BS, Blanco AI et al (2013) Salvage intensity modulated radiotherapy using endorectal balloon after radical prostatectomy: clinical outcomes. Int J Urol 20:1178–1183PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree