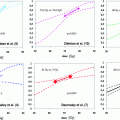
Risk group
Low
Intermediate
High
NCCN
T-Stage
cT1c + cT2a and
cT2b − 2c and/or
cT3 or
PSA
<10 ng/ml and
>10–20 ng/ml and/or
>20 ng/ml or
Gleason sum
<7
=7
8–10
T-Stage
cT1c − 2a and
cT2b and/or
cT2c − cT3 or
PSA
<10 ng/ml and
>10–20 ng/ml and/or
>20 ng/ml or
Gleason sum
<7
=7
8–10
2 International Commission on Radiation Units and Measurement (ICRU) Volumes Definition
ICRU 50, ICRU 62 and ICRU 82 provide a comprehensive definition of target volumes and organs at risk (OAR) in radiotherapy. The ICRU 50, published in 1993, presented the first concept of standardizing definitions of target volume delineation and organs at risk (International Commission on Radiation Units and Measurements 1993). The 1999 supplement (ICRU 62) introduced additional volumes that would change the extension of target volumes based on published data on internal organ motion and positioning uncertainties(International Commission on Radiation Units and Measurements 1999). In its latest modification from 2010 the ICRU report 83 takes into consideration new developments in imaging modalities as well as new radiation techniques and their influence on target volume definition(International Commission on Radiation Units and Measurements 2010).
The Gross Tumor Volume (GTV) comprises the macroscopic tumor extension. These are all tumor manifestations that are visible clinically as defined by the TNM system. Additional information is derived from imaging techniques such as ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), functional MRI or position emission tomography (PET), respectively. It may not only include the primary tumor but also lymph node metastases or distant tumor manifestations.
The Clinical Target Volume (CTV) is defined as the GTV plus a volume that is considered at risk of containing subclinical or microscopic disease. The extent of this margin differs widely among different tumor locations or different tumor histologies. Until now there is no consensus on how the risk of subclinical disease is defined but typically a probability of occult disease of 5–10 % is assumed to require treatment. The consideration of the consequences of failure, and the expected feasibility of salvage treatment furthermore influence the clinical judgment(International Commission on Radiation Units and Measurements 2010).
The Planning Target Volume (PTV) comprises the CTV with an additional margin derived from internal organ movement and patient set-up error. The PTV is the only target structure of which the size changes depending on the precision of the applied radiotherapy technique. Without any measures to determine the exact position of the prostate before or during the treatment fraction the CTV-PTV margin should cover the mentioned errors to provide a high probability of CTV coverage of the applied radiotherapy.
The Internal Target Volume (ITV) was introduced first in the ICRU report 62. The aim was to take into account possible changes of the CTV in terms of size shape and position. In prostate cancer these changes may be attributed to varying volumes of adjacent organs, namely rectum and bladder, which may cause altering CTV volumes.
The planning organ-at-risk volume (PRV) is directly associated with the ITV. Due to varying filling states of organs at risk the ITV allows for an improved determination of doses received by organs at risk during a treatment course. As volumes may vary substantially a precise measurement of these doses is not possible as the volume of an OAR may change not only between treatment fractions but also within a single fraction (inter- and intrafraction deviation). Furthermore a larger OAR volume may be advantageous as well as a distinct disadvantage in certain clinical situations. The rectum for instance may receive a significantly lower dose when fully extended by the use of an endrectal balloon, as the PTV positioned anteriorly will be treated in a constant position. In a systematic review a reduction of the rectal wall dose was shown in planning studies when an endorectal balloon was used. Yet there are no clinical studies to confirm whether rectal toxicities are reduced as well. (Smeenk et al. 2010). Furthermore an extended rectum may shift the prostate anteriorly and thus may result in an increased rectal dose when no image guided radiotherapy is applied.
Current randomized trials have used a variety of target delineations. Table 2 gives an overview of the available randomized trials on hypofractionation and dose escalation in primary prostate cancer radiotherapy. Obviously the target volume definition is different in each trial although prostate plus seminal vesicles were used as the CTV in most trials. The definitions of the PTV margins are variable as well, ranging from 3 to 20 mm.
Table 2
Summary of target volume definitions in the published Phase III trials
Phase III trials | CTV-definition | CTV-PTV margin |
|---|---|---|
Arcangeli et al. (2012) | prostate + SV | 10 mm uniform |
Lukka et al. (2005) | prostate | 15, 10 mm at PRI |
Pollack et al. (2006) | prostate + 9 mm inferiorly + proximal SV (intermediate risk); whole SV + LN (high risk) | conv. IMRT: 8, 5 mm at PRI hypo. IMRT: 7, 3 mm at PRI |
Norkus et al. (2009) | prostate + base of SV | 8–10 mm |
Yeoh et al. (2011) | prostate | 2D-RT: 15, 20 mm superior + inferior 3D-RT: 15 mm uniform |
MD Anderson (Kuban et al. 2008) | prostate + SV | ant. + inf.: 12.5–15 mm post. + sup.: 7.5–10 mm |
Dutch trial (Al-Mamgani et al. 2011) | prostate ± SV | Arm A: 10 mm (68 Gy) Arm B: 10 mm (68 Gy) Arm B: 5 mm (last 10 Gy) |
PROG96-09 (Zietman et al. 2005) | prostate + 5 mm | 7–10 mm |
GETUG 06 (Beckendorf et al. 2011) | 46 Gy: prostate + SV Arm A: prostate (24 Gy) Arm B: prostate (34 Gy) | 10 mm (5 mm posteriorly) |
MRC RT01 (Dearnaley et al. 2007) | low-risk: prostate + base of SV + 5 mm intermediate + high-risk: prostate + SV + 5 mm | 5–10 mm |
The following paragraphs will collect and sum up the available evidence on target volume definition.
3 Pathological Considerations for Prostate Cancer Clinical Target Volume Delineation
3.1 CTV Delineation in Low-Risk Prostate Cancer
Knowledge of the natural spread of prostate cancer is crucial for determination of reasonable clinical target volumes. The most comprehensive data on this issue can be derived from pathological studies. The tumor related bottleneck factors for CTV definitions are (a) the presence of extracapsular extension (ECE) and (b) seminal vesicle invasion (SVI).
In 1997 D’Amico et al. found in a retrospective analysis of 749 prostatectomy specimens that the risk of pathologic SVI as well as macroscopic extracapsular tumor extension is only 2 % in low risk patients. They furthermore found that the risk of PSA relapse is similar in patients undergoing radical prostatectomy or radiotherapy when patients without clinical risk factors were included (D’Amico et al. 1997b). These factors were defined as PSA <10 ng/ml, clinical stage <T2c and Gleason score <7, respectively. Further pathological studies confirmed these results (Kestin et al. 2002). Taking these findings into account there is no sensible reason to include the seminal vesicles into the CTV in patients with low risk prostate cancer. As the risk of ECE is similarly low it is obvious to delineate the prostate only as CTV defined by the visible boundaries on planning computed tomography or magnetic resonance imaging. An example of CTV delineation is shown in Fig. 1b.
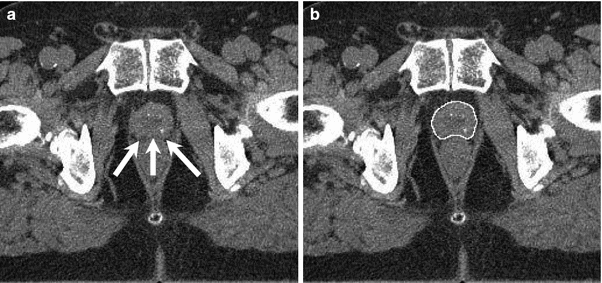

Fig. 1
Typical CT slice in the mid-gland region. Arrows indicate the fat layer at the prostate rectum interface (a). CTV contour of the same CT slice (b)
Does this hold true for patients with clinical risk factors?
3.2 CTV Delineation in Intermediate- and High-Risk Prostate Cancer
To shed light on this question it is useful to focus on the two most relevant factors of tumor recurrence after definitive therapy; namely extracapsular tumor extension and seminal vesicle involvement. It is well understood that the risk for these factors rises with increasing clinical risk factors. The question whether the degree of extracapsular extension may influence treatment outcome has been addressed by many author. Just recently van Veggel published an outcome study after radical prostatectomy focusing on the associated pathological ECE evaluation. Evaluation of the biochemical relapse rate of 134 patients according to different definitions of ECE revealed a significant association of the extent of ECE with the rate of biochemical relapse rates (van Veggel et al. 2011). Their results confirmed studies from various other authors (Epstein et al. 1993; Wheeler et al. 1998; Davis et al. 1999). To be able to apply these results to target volume definition in prostate cancer radiotherapy it is necessary to evaluate the specific amount of extracapsular tumor extension. In the largest published series The et al. found that among all prostatectomy specimens with extracapsular tumor extension only 2.8 % show an extension of more than 5 mm from the capsule (Teh et al. 2003). Several pathological studies have confirmed that more than 90 % of all patients who present with ECE have a radial tumor extension from the capsule of less than 4–5 mm (Davis et al. 1999; Schwartz et al. 2007; Sohayda et al. 2000).
CTV definition of the prostate gland plus an additional circumferential margin of 5 mm would thus cover more than 90 % of tumor extensions outside the prostate. At the prostate rectum interface the anterior rectal wall represents a solid boundary for tumor cells so that the CTV can be reduced posteriorly. An example is shown in Fig. 2.
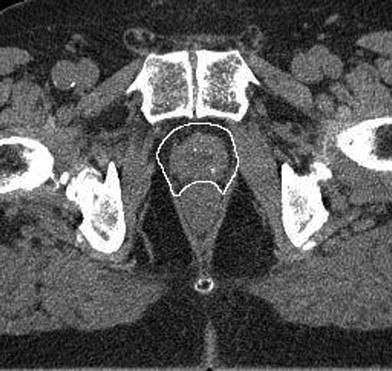

Fig. 2
Contouring of the prostate plus a 5 mm margin to include the possible periprostatic extension in patients with risk factors
Kestin and colleagues reviewed 344 prostatectomy specimens with regard to SVI. They found that the tumor never involves the whole SV; the most distant cancer found was 1.5 cm apart from the SV tip. Including the proximal 2 cm of the SV into the CTV would include 90 % of pathological involved seminal vesicles. In terms of certainty of CT definitions of the seminal vesicle compared to pathological measurements they furthermore found that the two methods are in well agreement (Kestin et al. 2002). Given that 38 % of all patients showed at least one high risk feature it seems advisable to include the proximal 2.0–2.5 cm of the seminal vesicles into CTV in order to cover more than 90 % of all SVI. Finally only 1 % of low-risk patients had a SVI which confirms not to include SVs into CTV in this patient group. With each prognostic high risk factor the rate of SVI rises from 15 % with one high risk factor, 28 % with two and 58 % with three high risk factors respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree