Chapter 11 Volumetric Assessment
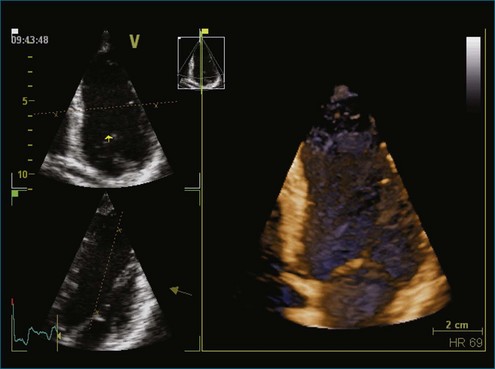
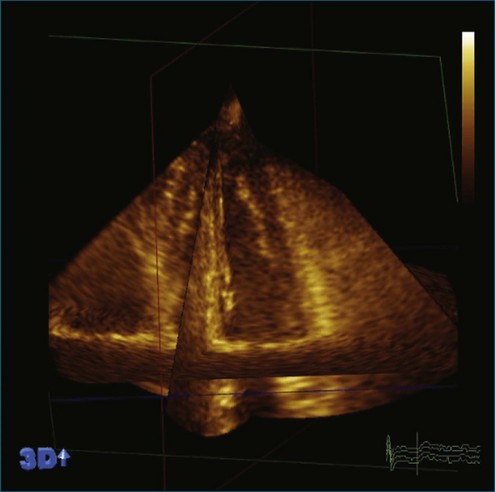
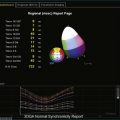
The rationale for the use of three-dimensional echocardiography (3DE) in a clinical setting is growing stronger. The four main areas in which the value of 3DE has been investigated include (1) the analysis of cardiac volumes and left ventricular mass, (2) ischemic heart disease, (3) congenital heart disease, and (4) valvular pathology. Although various versions of 3DE have been in use since the early 1970s, “live” or real-time 3DE (RT3DE) has only been in use since the early 2000s (Figures 11-1 and 11-2; Videos 11-1 and 11-2).1
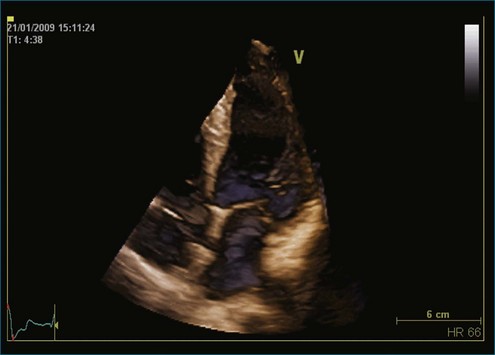
Figure 11-2 Example of a real-time three-dimensional image from the apical view (see Video 11-2).
(Courtesy EchoPAC-PC, GE Vingmed.)
3DE can display, in real time, the views and motions of deeper cardiac structures, which are unavailable by two-dimensional echocardiography (2DE), and therefore is capable of providing superior diagnostic information (Figures 11-3 to 11-5; Videos 11-3 to 11-5). This is particularly advantageous given the complex spatial relations of cardiac structures, especially in the fields of acquired valvular disease, atrial and ventricular septal defects, and left ventricular remodelling.2–4 It can also display exact assessments of left atrial volume.5,6 3DE is particularly well suited for volumetric analysis of the right ventricle since it has such an irregular shape. The ability of 3DE to simulate surgical views helps facilitate vital surgical decisions such as the accurate assessments of the effect of percutaneous balloon valvuloplasty and the function of prosthetic valves and septal occluders. Finally, 3DE may help in making more accurate qualitative diagnosis and in the classification of congenital heart disease.7
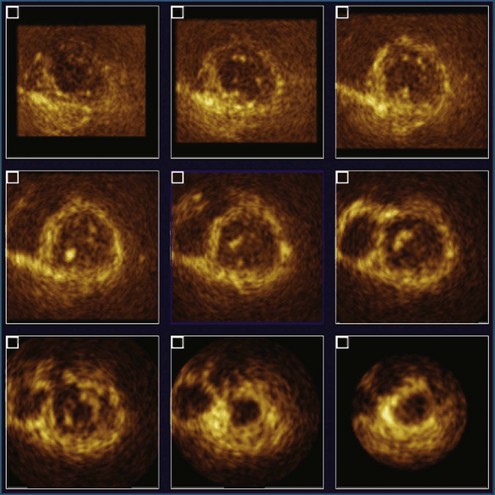
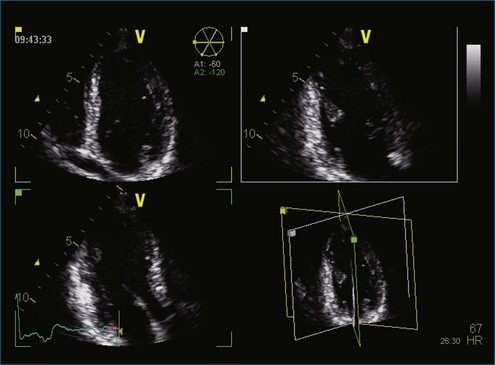
Figure 11-4 Example of nine-slice view of a full-volume three-dimensional image (see Video 11-4).
(Courtesy Philips Healthcare, Andover, MA.)
Qualitative and Quantitative Problems with Clinical Two-Dimensional Echocardiography Today
Quantification of left ventricular volumes and ejection fraction (EF) is an important aspect of cardiac evaluation in all cardiac disorders. Indeed, assessment of left ventricular function is the most common indication for echocardiography. The serial assessment of left ventricular function frequently is used to guide therapy. However, repeated measurements are prone to variation because of poor image quality, geometric issues related to volume and mass calculations, performance measurements from off-axis cuts, and variations in ventricular loading.8
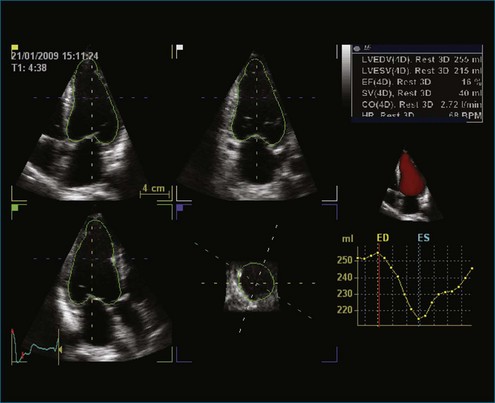
EF is a simple numeric value that reflects left ventricular function; however, it is strongly influenced by loading conditions and, in many cases, does not correlate with the patient’s symptoms. More importantly, it has limited test-retest reliability.9 Subjective visual or “eye-ball” assessment of left ventricular EF (LVEF) often is performed in clinical practice but can be misleading because of irregular heart rhythms, when the left ventricular cavity is very large or very small, or when the heart rate is very low or very fast.10 Motion mode (MM) has been used for many decades to calculate the fractional shortening and EF from the left ventricular diameters at end diastole and end systole; however, these values have large variations because of angle dependency.11 Studies have shown that Simpson’s biplane calculation of EF can vary up to 4.1% between readers.12,13 This variability is caused by the complex geometric assumptions and potential problems with image foreshortening presented by 2DE calculation of EF. Previous work by King et al11 has shown the importance of cut planes on cardiac measurements. In their study, 2DE did not achieve consistent optimal positioning of standard imaging views, resulting in a high percentage (93%) of off-axis images with resultant variations in left ventricular measurements (9%).14 Neither MM nor the Simpson’s biplane technique take into account the regional variation of the whole left ventricular volume, which may occur due to myocardial infarction. Similar findings have been reported for left ventricular mass. The smallest change of mass that can be detected with 95% confidence is 59 g, which compares with an average 20 to 40 g/year change in most antihypertensive therapy trials.15
Consequently, cardiac magnetic resonance imaging (MRI) has been proposed as a more desirable alternative for left ventricular assessment, especially in clinical trials, because of its excellent image quality and high spatial resolution.16 Given this, cardiac MRI has become the gold standard for left ventricular volumes, EF, and left ventricular mass. However, expense, patient intolerance (e.g., claustrophobia, noise), a relative contraindication in patients with cardiac devices, and lack of portability have limited the use of this modality in routine clinical practice.
Despite the technical limitations of 2DE, it remains the most widely used noninvasive technique for the measurement of left ventricular size and function. These limitations may be overcome with the use of 3DE, which has less test-retest variation, better reproducibility, and better accuracy compared with 2DE.9,17–21
Assessment of Left Ventricular Volumes and Ejection Fraction
In the early 1990s, volumetric 3DE was developed by Duke University using a sparse matrix array transducer. This technique is based on the concept that the heart would fit into a pyramidal dataset and does not rely on the transducer movement or sequential capturing. Although the output is known as “real-time output,” it actually consists of multiple 2D images displayed simultaneously.22 Other previous 3D transducers used a modified 2D probe that had elements arranged in a single line, in which multiple windows and planes were needed to reconstruct an image. These volumetric and reconstructed volumes were found to have improved accuracy for left ventricular volumes.
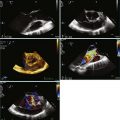
These early techniques formed the basis of RT3DE. Current matrix transducers use a dense array, rather than the previous sparse array arrangement, and reportedly have more than 3000 elements.23 RT3DE uses 256 firing elements in the form of a grid instead of a line, which enables acquisition of an online 3D volume of ultrasound data (Figures 11-6 to 11-10; Videos 11-6 to 11-10).
Figure 11-6 Example of normal left ventricular systolic function and wire frame over the cardiac cycle (see Video 11-6).
(Courtesy TomTec, Munich, Germany.)
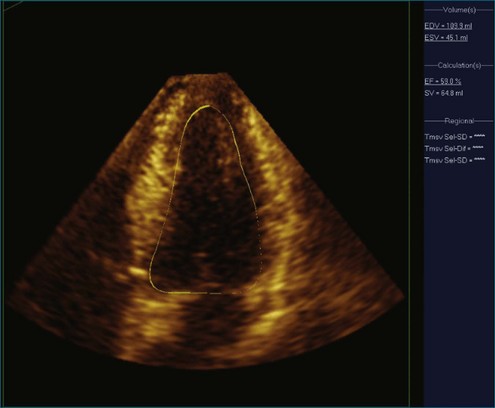
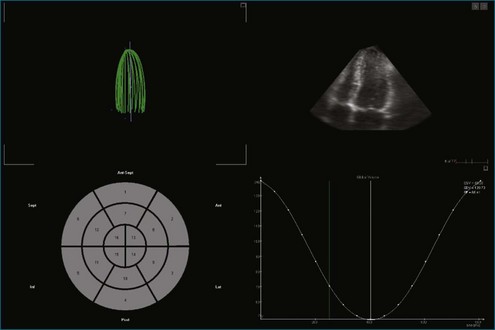
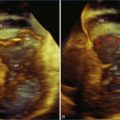
Figure 11-8 Example of poor left ventricular systolic function and the 16-segment model (see Video 11-8).
(Courtesy TomTec, Munich, Germany.)
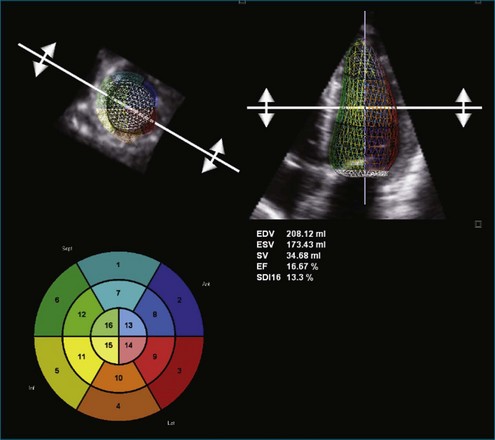
Figure 11-9 Example of poor left ventricular systolic function and a time volume curve (see Video 11-9).
(Courtesy EchoPAC-PC, GE Vingmed.)
Online 3DE allows volumetric quantification using a biplane or triplane technique.12 3DE has the advantage of accurate delineation of the true long-axis length of the ventricle, thus increasing the accuracy of Simpson’s guided biplane measurements (see Figures 11-4 and 11-5; see Videos 11-4 and 11-5). For 2DE, the accuracy of left ventricular volumes by Simpson’s method depends on the apical four-chamber and two-chamber lengths being nearly equal. Since the geometric assumptions of the 2DE calculations depend on the accuracy of ventricular lengths, foreshortening will result in underestimation of the cross-sectional area.
Recent advancements in 3DE technology have allowed for faster assessment in full left ventricular volume measures because of the semiautomated endocardial edge detection. Online measurement of left ventricular volumes is feasible and more accurate than with 2DE.24
Despite semiautomated measuring techniques, 3DE has been found to underestimate left ventricular volumes compared with MRI measures. The major shortcoming of 3DE relates to the image quality of the currently obtainable images (Figure 11-11; Video 11-11). The lower line density and frame rate increase the difficulties in discriminating the endocardial border and are potential contributors to inaccuracies in the measurement of left ventricular volumes. In addition, even with the volume dataset, the apex can be difficult to visualize. However, more advanced software that allows the 3D volume datasets to be aligned such that the apex is optimally visualized has become available. The difficulty in endocardial border visualization often can be overcome by the use of contrast imaging, namely left ventricular opacification (LVO). Studies have demonstrated increased accuracy using LVO for both 2DE and 3DE calculation of left ventricular volumes and EF (Figure 11-12; Videos 11-12 and 11-13).25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree