Whole-body magnetic resonance neurography (WBMRN) is a radiation-free imaging modality enabling comprehensive evaluation of the peripheral nervous system from the skull base to the knee. Using advanced sequences like isotropic high-resolution 3-dimensional short-tau inversion recovery and diffusion tensor imaging, WBMRN provides high-resolution visualization of nerve anatomy and pathology, offering insights into structural and microstructural abnormalities. It aids in diagnosing and monitoring conditions such as Charcot-Marie-Tooth disease, neurofibromatosis, and chronic inflammatory demyelinating polyneuropathy. This article outlines the imaging approaches used widely for whole-body MR imaging, and discusses indications, imaging differences, and role of WBMRN for evaluation of various neuropathic disease conditions.
Key points
- •
Whole-body magnetic resonance neurography (WBMRN) is a radiation-free, noninvasive imaging technique for comprehensive evaluation of the peripheral nervous system.
- •
It is effective in diagnosing and monitoring neuropathic conditions such as Charcot-Marie-Tooth disease and neurofibromatosis.
- •
The use of biomarkers like apparent diffusion coefficient and fractional anisotropy provides valuable quantitative insights into nerve integrity and disease progression.
- •
Technological advancements, including artificial intelligence integration, enhance diagnostic precision and reduce imaging times in WBMRN.
| ADC | apparent diffusion coefficient |
| AI | artificial intelligence |
| BP | brachial plexus |
| CIDP | chronic inflammatory demyelinating polyneuropathy |
| CMT | Charcot-Marie-Tooth |
| CT | computed tomography |
| DETECT | Dual Echo T2-weighted acquisition with Enhanced Conspicuity of Tumors |
| DTI | diffusion tensor imaging |
| DWI | diffusion-weighted imaging |
| DWIBS | diffusion-weighted imaging with/without background suppression |
| FA | fractional anisotropy |
| FSE | fast spin echo |
| GRE | gradient echo |
| HNPP | hereditary neuropathy with liability to pressure palsy |
| IP | in-phase |
| LS | lumbosacral |
| MIP | maximum intensity projection |
| ML | machine learning |
| MMN | multifocal motor neuropathy |
| NF | neurofibromatosis |
| NF1 | neurofibromatosis type 1 |
| OP | out-of-phase |
| PNST | peripheral nerve sheath tumor |
| REiNS | Response Evaluation in Neurofibromatosis and Schwannomatosis |
| SNR | signal-to-noise ratio |
| STIR | short-tau inversion recovery |
| tDV | total diffusion volume |
| TE | echo time |
| TI | inversion time |
| TR | repetition time |
| TSE | turbo spin echo |
| T1W | T1-weighted |
| WBMRI | whole-body MR imaging |
| WBMRN | whole-body magnetic resonance neurography |
| 2D | 2-dimensional |
| 3D | 3-dimensional |
Introduction
Whole-body magnetic resonance neurography (WBMRN) is a novel radiation-free modality for the evaluation of peripheral nerves from skull base to the knee level using a combination of several high-resolution sequences including isotropic 3-dimensional (3D) MR imaging and diffusion-weighted imaging (DWI). It allows multiplanar imaging of the plexuses and peripheral nerves for the assessment of nerve signal changes, caliber, and structural abnormalities along the entire peripheral nervous system, thus providing a detailed map of neuropathic involvement that can aid in diagnosis, treatment planning, and progression tracking. Initially proposed by Yamashita and colleagues using multistation planar DWI in 2009, imaging techniques have evolved over the years with new hardware and software approaches, and incremental refinement of the MR imaging sequences. This article will outline the imaging approaches used widely for whole-body MR imaging (WBMRI) and discuss the indications, imaging differences, and role of WBMRN for evaluation of various neuropathic disease conditions.
Whole-Body MR Imaging: a Comprehensive Modality with Wide-Ranging Applications
WBMRI represents an advanced imaging modality, increasingly utilized for its ability to deliver comprehensive imaging of the whole-body without the risks associated with ionizing radiation. In contrast to conventional MR imaging techniques, WBMRI combines high-resolution anatomic visualization with functional data—primarily through DWI—enabling detailed assessment of soft tissues, bone marrow, and visceral organs in a single, integrated examination. This multiplanar approach enhances diagnostic accuracy, allowing radiologists to identify and monitor pathologies, such as metastatic disease with precision, providing a valuable alternative or adjunct to PET/computed tomography (CT) in both staging and therapeutic response settings. Technological advancements, such as multichannel surface coils, fast parallel imaging, and moving-table acquisition, now facilitate faster and more expansive imaging coverage from head to toe, making WBMRI increasingly accessible and feasible for routine clinical applications. The common indications where WBMRI has been recommended include neurofibromatosis (NF), schwannomatosis, Li-Fraumeni syndrome; early-onset cancerous lesions associated with germline mutations in p53 gene, and multiple myeloma. ,
In the next section, the evolution of clinical WBMRI sequences is discussed, followed by further modifications to the sequences that enable whole-body neurography.
Clinically applied whole-body MR imaging sequences
As outlined earlier, WBMRI is increasingly recognized as a powerful imaging modality for its comprehensive coverage, nonionizing radiation-free nature, and detailed tissue characterization. This renders WBMRI especially valuable for oncological evaluation and neurologic to inflammatory disease assessment, due to the high sensitivity and specificity afforded by multiparametric imaging sequences. WBMRI is variably performed in different settings, usually integrating a set of anatomic and functional sequences—such as short-tau inversion recovery (STIR), T1-weighted (T1W) imaging, DWI with/without background suppression (DWIBS), and Dixon imaging—to deliver a robust diagnostic platform. , Each sequence contributes unique diagnostic strengths, together enhancing the visualization of diverse pathologies that span the body. The imaging is performed using a set of large multichannel torso coils linked tightly together physically as well as digitally to the spine coils. Depending upon the height of the patients, 2 to 4 sets of such coils may be required.
The multiparametric approach in WBMRI, combining these distinct imaging sequences, provides a comprehensive diagnostic toolkit that enables radiologists to assess various systemic diseases accurately. Each sequence brings a unique diagnostic perspective, whether through detailed anatomic delineation, functional imaging insights, or tissue-specific contrast, and together they improve the sensitivity and specificity of WBMRI for diverse pathologies. By integrating these sequences, WBMRI meets the demands of modern diagnostic imaging with a comprehensive view across a wide array of clinical conditions.
Anatomic whole-body MR imaging sequences
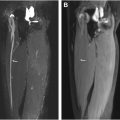
The STIR sequence is a cornerstone of WBMRI, particularly valued for its ability to suppress fat signals, which enhances the visualization of edematous and infiltrative lesions. STIR is a time-dependent inversion recovery sequence combined with a fast spin echo/turbo spin echo (FSE/TSE) acquisition and thus can also be used in patients with metal in the field of view. It provides improved fat suppression at lower magnetic field strengths than frequency selective fat suppression imaging such as Spectral Presaturation with Inversion Recovery (SPIR) or Spectral Adiabatic Inversion Recovery (SPAIR). The optimal and recommended repetition time (TR), echo time (TE), slice thickness (Δ z ), interslice gap, and inversion time (TI) are 3000 to 4000 ms, 20 to 30 ms, 4 to 5 mm, 0% to 10%, and 150 ms/220 ms for 1.5 T and 3T, respectively. This sequence is highly sensitive to pathologic changes within the soft tissues and the bone marrow, making it indispensable in detecting early inflammatory and neoplastic conditions. For example, in the evaluation of NF syndromes, STIR provides clear delineation of nerve sheath tumors and helps quantify tumor burden, thereby supporting both diagnosis and monitoring of disease progression ( Fig. 1 ). In cases of myopathy or systemic inflammation, STIR effectively reveals muscle inflammation patterns, as demonstrated in polymyositis, where it captures diffuse muscle group involvement, aiding both initial assessment and longitudinal tracking. Furthermore, STIR’s ability to detect lesions with high water content or edema makes it invaluable in assessing musculoskeletal conditions, where early inflammatory changes may not be evident on other sequences, such as in chronic recurrent multifocal osteomyelitis. ,

Because of its ability to suppress fat and highlight structures with higher water content, STIR is also valuable for delineating nerves and muscle denervation even in regions of the body with challenging anatomy, such as the brachial plexus (BP).
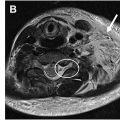
T1W imaging is often paired with STIR in WBMRI protocols for its superior anatomic clarity and high spatial resolution, which are crucial for differentiating tissue types and assessing lesion composition. The optimal and recommended TR, TE, Δ z , and interslice gap of an FSE/TSE-based T1W acquisition are 600 to 900 ms, 6 to 9 ms, 4 to 5 mm, and 0% to 10%, respectively. FSE/TSE-based T1W imaging is often acquired in either 2-dimensional (2D) or 3D and is useful for delineating pathologies in bone marrow. A T1W sequence also provides a reliable baseline for contrast studies, allowing radiologists to evaluate the degree of contrast enhancement, which is often indicative of vascularity and potential malignancy. However, on 1.5T and 3T scanners, it is ideal to obtain a 3D T1W sequence, as a gradient echo (GRE) sequence with or without Dixon manipulation and with or without subtraction for faster imaging and volumetric assessment; a 3D TSE sequence is also possible, although requires a longer scan time. The use of such 3D sequences is shown in Fig. 2 , and the Dixon technique will be further discussed later. The images can be thus reconstructed in multiple planes for optimal lesion and anatomy assessment. The optimal and recommended TR, TE, and Δ z are 8 to 9 ms, 3 to 4 ms, and 1.5 mm, respectively. Because these are acquired as 3D images, the interslice gap will always be 0%. Due to volumetric 3D acquisitions with higher signal-to-noise ratio (SNR) on higher field scanners, it is faster to obtain 3D images with reconstruction into thinner slices than to obtain thin-slice T1W images at shorter scan times.

In oncological applications, T1W imaging is vital for anatomy assessment, delineating tumor boundaries, determining intralesional fat, and identifying bone marrow involvement, particularly when combined with postcontrast imaging to enhance lesion characterization. This sequence is commonly used to assess both primary and metastatic disease across a wide range of cancers, including skeletal and soft tissue metastases and is useful for the assessment of response to treatment, such as for determining fatty metamorphosis of axial spondyloarthropathy lesions and multiple myeloma lesions. T1W images are also valuable in assessing nerves, as they can highlight areas of nerve damage with loss of normal architecture or fibrosis and inflammation. Particularly in conjunction with contrast enhancement, T1W images can be useful for identifying conditions, such as nerve compression.
Functional whole-body MR imaging sequences
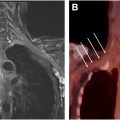
DWI , especially when implemented as DWI with background suppression (DWIBS), adds a functional dimension to WBMRI by providing insights into cellular density. DWI is highly sensitive to microstructural changes that occur in response to cellular proliferation or necrosis, making it exceptionally useful in detecting malignant lesions, which are often present with restricted diffusion due to increased cellularity. The optimal parameters based on our clinical practice include TR, TE, Δ z , interslice gap, and diffusion moment ( b -values) of 6000 to 8000 ms, 60 to 70 ms, 5 mm, 0% to 10%, and b = 50,400,800 s/mm 2 , respectively. , DWIBS is particularly advantageous in WBMRI because it suppresses background signals, enabling clearer visualization of lesions even in complex anatomic regions. This sequence has proven invaluable in oncologic applications, where it assists in detecting and staging metastases, treatment response, and evaluating systemic inflammatory conditions. The ability of DWI to provide apparent diffusion coefficient (ADC) values further enhances its utility, as ADC values can correlate with tumor aggressiveness, offering a quantitative marker for disease characterization. , , Thus, DWIBS complements anatomic sequences by highlighting both visible and relatively inconspicuous disease due to better background suppression. However, the DWI sequence can be degraded by geometric distortions, motion, and/or ghosting artifacts. Using inversion-recovery-based fat suppression like STIR or SPIR, high bandwidth to reduce echo spacing, keeping the lowest possible TE, and allowing the patient to be comfortable during imaging allows the best possible DWI with acceptable imaging times of 5 to 6 minutes per station (see Fig. 1 ).
Advances to whole-body MR imaging sequences
Fast parallel imaging and new coil and software technologies have reduced the time of WBMRI from more than 1 hour of imaging to below 45 minutes. Artificial intelligence (AI) approaches have further increased SNR and reduced artifacts for different sequences described above for WBMRI. In addition, moving table technology and auto-alignment of imaging planes are further advances aiding in the reduction of acquisition times. Furthermore, auto-analysis of lesions is possible with deep learning algorithms reducing the interpretation times.
Dixon-based imaging is becoming popular in WBMRI protocols for water-fat separation, enhancing the differentiation of tissues based on fat content. This sequence is particularly advantageous in musculoskeletal imaging, where it can effectively distinguish between fatty and nonfatty tissues, a capability crucial for identifying soft tissue lesions, bone marrow abnormalities, certain types of fat-containing tumors, and post-treatment response with calculation of fat fraction of the lesion. Dixon imaging achieves high contrast between tissues and reduces the scanning time without compromising diagnostic quality, providing an efficient alternative to traditional T1W and STIR sequences in WBMRI. , The parameters are similar to the ones described for 3D T1W imaging. The capability of Dixon imaging to generate multiple contrast images in a single acquisition enhances workflow efficiency, especially in high-throughput environments where comprehensive body assessments are routine. Integration of Dixon with single-shot FST/TSE (or half-fourier acquisition single-shot turbo spin-echo [HASTE]) sequences provides a time-efficient acquisition of water-only, fat-only, in-phase (IP), and opposed-phase images. Thus, it can prudently replace the conventional FSE T1W and STIR imaging while providing higher SNR and less pulsation artifacts than STIR imaging. In addition, Dixon imaging has been widely used for body fat and muscle composition and sarcopenia imaging. ,
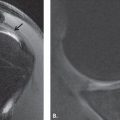
In our set-up, we have developed a less than 10-minute WBMRI protocol based on T2W Dixon imaging, which can produce equivalent WBMRI to above-described sequences for the purpose of a metastatic evaluation. This dual-echo T2weighted imaging (T2W) acquisition for enhanced conspicuity of tumors (DETECT) acquires 4 images, IP and out-of-phase (OP) at a short and a long TE using single-shot turbo spin echo. The IP/OP images at the short and long TEs are reconstructed using the standard Dixon and shared-field-map Dixon reconstruction, respectively, for robust fat-water separation. An adaptive complex subtraction between the 2 TE water-only images achieves fluid attenuation. This advanced imaging provides good lesion conspicuity with robust fat and fluid suppression ( Fig. 3 , Table 1 ).

| Sequence | Purpose | Benefits | Common Applications |
|---|---|---|---|
| STIR | Excellent fat suppression, edema detection | High sensitivity for inflammation, tumor burden visualization | Inflammatory myopathies, neurofibromatosis, tumor load assessment |
| T1W | Anatomic clarity, tissue differentiation | Good structural detail, baseline for contrast studies | Bone marrow involvement, tumor delineation |
| DWI/DWIBS | Functional imaging, cellular density measurement | High sensitivity to cellular changes, background suppression | Metastasis detection, inflammation, systemic cancer screening, and treatment response |
| Dixon Imaging | Water-fat separation | Enhanced contrast and high SNR imaging, efficient scanning | Multimap (IP, opposed-phase, selective water and fat) imaging, soft-tissue and bone lesion characterization, fat fraction assessment |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree