Clinical Presentation
A newborn male presents with flattening of the nasal bridge, mild respiratory distress, and unusually short, broad distal phalanges of the hands and feet.

Figure 117A

Figure 117B
Radiologic Findings
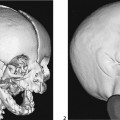
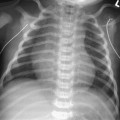
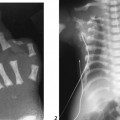
The AP radiograph of the hand shows abnormal morphology of the second through fourth distal phalanges, with distal broadening, proximal narrowing, and shortening of the tufts (Fig. 117A1), with puncta at the distal phalangeal epiphyses (Fig. 117A2, arrowheads). Dramatic calcification of the tracheal cartilaginous rings is seen on the oblique neck and upper chest radiograph (Fig. 117B, arrowheads).

Figure 117C

Figure 117D

Figure 117E
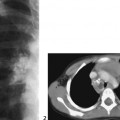
The lateral view of the vertebral column shows several coronal clefts (Fig. 117C, arrows) and puncta at the tips of the spinous processes (Fig. 117C, arrowhead). The pelvic radiograph demonstrates characteristic calcific puncta within the triradiate cartilages (arrows) and greater trochanteric apophyses (arrowheads), as well as dramatic paraspinal puncta (Fig. 117D). CT of the nasal passage shows puncta within the nasal alae and septal cartilage (arrows), as well as severe choanal narrowing (Fig. 117E).
Diagnosis
Chondrodysplasia punctata (CDP), brachytelephalangic subtype (CDP-BT)
Differential Diagnosis
The most significant entities in the differential diagnosis for stippled epiphyses in the newborn are:
- Conradi-Hünermann syndrome (CDP-X-linked dominant, CDP-X-linked recessive) (Fig. 117F)
- Rhizomelic chondrodysplasia punctata (CDPR) (Fig. 117G)
- I-cell disease (shown in an older child, Fig. 117H)
- GM1 gangliosidosis (Fig. 117I)
- Zellweger syndrome (Figs. 117J and 117K)
- Antenatal warfarin exposure
- Brachytelephalangic chondrodysplasia punctata
Discussion
Background
For most radiologists, the differential diagnosis of punctate, stippled epiphyses is limited to a few entities, usually Conradi-Hünermann syndrome, Zellweger syndrome, and “chondrodysplasia punctata.” However, to paraphrase Dr. Poznanski, CDP is a radiologic sign, not a distinct disease entity (Poznanski 1994). Indeed, the list of conditions associated with epiphyseal punctate calcifications is actually quite extensive and includes skeletal dysplasias, isolated single gene defects, inborn errors of metabolism, chromosomal trisomies/deletions, teratogenic exposures, infections, endocrinopathies, and maternal disorders (Table 117A).
Etiology
The pathogenesis of epiphyseal and extraskeletal cartilaginous calcific stippling has been best studied in a rat model of warfarin embryopathy. Rats administered warfarin produce offspring with premature punctate calcification of the nasal septum and snout, with resultant shortening and narrowing of the nasal passages. They also demonstrate abnormal calcific bridges within the physes of long bones and vertebrae. Within the nasal septum and epiphyses, chondrocytes are decreased in number and are abnormally clustered, demonstrating a disorganized pattern with premature punctate calcification within their surrounding matrix. These observations were confirmed by Barr and Burdi (1976) in their study of a human fetus with warfarin embryopathy.
Similar findings have been reported in histologic analysis of human patients with CDPR. These patients demonstrate clumps of disorganized epiphyseal chondrocytes separated by abnormal fibromuscular tissue containing punctate calcifications. Gaulier and colleagues (1987) reported similar findings in a patient with classic Conradi-Hünermann syndrome (CDP-XD); this patient also showed intramural calcification within epiphyseal vascular channels. Two reports of auricular biopsies in patients with Keutel syndrome (Keutel, 1972) describe multifocal, abnormal, progressive cartilaginous ossification. However, there has been no evidence to suggest that focal cartilaginous hemorrhage causes epiphyseal stippling in any of these disorders.
Matrix GLA protein (MGP) is produced by cartilage and is found in all noncalcified cartilaginous tissues. When properly carboxylated, its function is to inhibit hydroxyapatite formation within cartilage. Inactive, decarboxylated MGP is found in the nasal and epiphyseal cartilage of warfarin-treated rats; perhaps poor or absent MGP carboxylation represents the end result of numerous divergent metabolic pathways that produce the phenotype of chondrodysplasia punctata.
| Types | |
| Single Gene Disorders and Skeletal Dysplasias | Acrodysostosis |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree