TABLE 5-1
Indications for Breast Ultrasound
Evaluation of a palpable mass or other signs/symptoms in the breast
Evaluation of abnormalities on other imaging studies (such as mammography or MRI)
Initial imaging of a palpable mass in women under 30 (who are not at high risk for breast cancer) and in lactating/pregnant women
Evaluation of problems with breast implants
Evaluation of lesions suspicious for malignancy (microcalcifications or architectural distortion) in the setting of dense fibroglandular tissue
Guidance of breast biopsy or interventional procedure
Treatment planning for radiation therapy
Screening for occult cancer as a supplement to mammography in certain populations of women who are not candidates for MRI
Identification and biopsy of abnormal axillary lymph nodes
Breast ultrasound can be divided into two categories: targeted and nontargeted. Targeted ultrasound is used to identify a specific lesion or region in the breast, but does not scan the entire breast. Palpable superficial lesions may be better seen on ultrasound than on mammogram.4 Non-targeted ultrasound is used to evaluate the entire breast in the presence of symptoms such as nipple discharge, silicone implant rupture, and known breast cancer or axillary metastasis in the presence of a negative mammogram.5 Whole breast ultrasound is not routinely performed for breast cancer screening in the general population due to the low sensitivity; however, certain populations such as women with dense fibroglandular breasts or high-risk groups may benefit from sonographic screening.2
In the setting of a known breast cancer, ultrasound can be helpful for complete characterization of a lesion for surgical planning and tumor staging. Ultrasound may also help to visualize multifocal or multicentric carcinoma, and is frequently used to scan the axilla to check for the presence of suspicious lymph nodes. As MRI is used more frequently in breast cancer patients as well as for screening in high-risk populations, post-MRI ultrasound often identifies lesions and facilitates biopsy under ultrasound guidance.2
Intraoperatively, ultrasound can be useful to guide surgery.6 Several studies have suggested that use of intraoperative ultrasound decreases reexcision rates by guiding margin excision around a lesion at the time of surgery. Intra-operative wire localization under ultrasound guidance can be performed by the surgeon for nonpalpable lesions, which saves time and is more comfortable for the patient. Ultrasound can also guide minimally invasive therapeutic interventions such as cryotherapy and radiofrequency ablation.7,8
Clear documentation is necessary for many reasons including billing and compliance, as well as to ensure accurate duplication of the study if additional procedures will need to be performed. Correct documentation includes several components (Table 5-2).
TABLE 5-2
Documentation of an Ultrasound Image
Examination date
Patient’s first and last name
Medical record number and/or date of birth
Designation of right or left breast
Anatomic location using clock face notation or a labeled diagram of the breast. Transducer orientation and distance from the nipple to the abnormality should be documented
Sonographer/physician’s identification number, initials, or other symbols
NORMAL ANATOMY
The form and function of the breast change over time and depend on hormonal factors occurring during puberty, pregnancy, and menopause. During reproductive years, the mature breast consists of 15–20 lobes of branched ducts, which radiate from the nipple and divide peripherally into lobules. Each lobe has numerous lobules and small branch ducts that join together to form larger ducts, which then form a main subareolar duct that drains the whole lobe. The lobes are different sizes and sometimes overlap one another. The gland is surrounded by support structures made up of subcutaneous connective tissue, which forms septa between lobes and lobules.
The terminal ductolobular unit (TDLU) makes up the functional unit of the breast. It consists of a single lobule and the terminal duct. The number of TDLUs varies between patients, and also changes in a single patient’s breast for reasons including age, parity, body habitus, and hormonal factors. During pregnancy and lactation, women often have very rapid proliferation of TDLUs.2
Anatomy of the breast from superficial to deep consists of skin, subcutaneous fat, anterior fascia, breast parenchyma (containing ductal and lobular structures), retromammary fascia, retromammary fat, pectoralis muscle, ribs, and pleura.6 Cooper’s ligaments are present within the breast parenchyma and subcutaneous fat layers. The tail of Spence (or axillary segment) of the breast extends toward the axilla. The nipple is not perfectly centered on the breast, but lies just medial and inferior to the center. This subsequently allows for the upper outer quadrant of the breast to contain more volume, which may account for the greater number of breast lesions seen in the upper outer quadrant. The nipple and areola are covered by pigmented epithelium and contain smooth muscle, which can contract and cause acoustic shadowing during ultrasound.2
Lymphatic drainage of the breast is extensive, and is the route by which most breast cancers spread. The breast first drains into superficial lymphatic channels, which travel from the subareolar plexus along the major ducts, then along efferent veins to adjacent nodal beds. The majority of the breast drains primarily to the axilla, including the external mammary, axillary, and central nodal groups within the axilla. More distal groups involved in advanced disease include the interpectoral nodes, subclavicular nodes, and supraclavicular nodes. The scapular and subclavicular nodes are also contained within the axilla. Medial lymphatics also drain directly into the internal mammary nodes. The presence, level, and number of axillary lymph nodes involved are important in prognosis, as well as treatment, of breast cancer. Intramammary lymph nodes may also be present, and are most commonly located in the axillary tail but may be located anywhere within the breast.
Blood supply to the breast comes from perforating branches of the internal mammary artery along the medial aspect of the breast, intercostal perforators, and lateral thoracic and thoracoabdominal branches of the axillary artery. Venous drainage is variable in the breast. The deep veins tend to run along the same course as the arterial supply. In general, the superficial veins do not have corresponding arteries; rather the venous drainage runs with the lymphatic drainage.2
NORMAL APPEARANCE OF ULTRASOUND
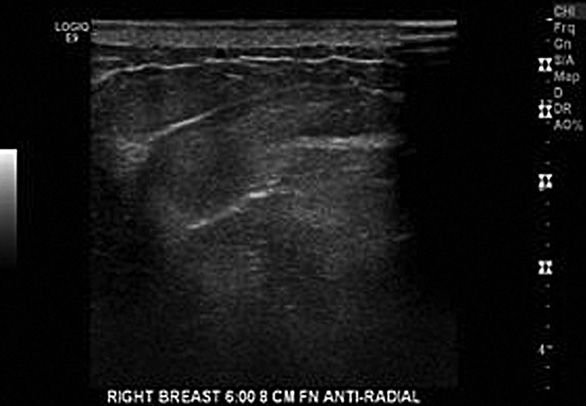
The skin appears as a hypoechoic line at the top of the image, which becomes thicker toward the nipple-areola complex. A bright hyperechoic linear echo can be seen between the dermis and subcutaneous fat planes. The skin appears as a 1- to 3-ml isoechoic to hyperechoic strip sitting on top of this echo. The skin can be thickened on ultrasound secondary to scarring, infection, radiation-induced dermatitis, and fat necrosis (Figure 5-1).2,9
The subcutaneous zone lies between the skin and anterior mammary fascia. It includes subcutaneous fat, Cooper’s ligaments, and blood vessels. The subcutaneous fat is between the skin and breast parenchyma. The amount of subcutaneous fat present in a breast varies with age, parity, and body habitus. The subcutaneous fat appears as a hypoechoic region below the skin, and thins out toward the nipple-areola complex. Cooper’s ligaments are linear hyperechoic structures arising from the breast parenchyma, which can be seen throughout the glandular tissue as well as the subcutaneous fat. Blood vessels, ectopic ducts, and lobules may be present in the subcutaneous region as well. Most sonographic lesions seen in the subcutaneous zone arise from the skin or subcutaneous fat rather than breast tissue. These lesions are not specific to the breast, and include epidermal inclusion cysts, lipomas, hemangiomas, and sebaceous cysts.2,10
The mammary zone is also called the glandular region, and is composed of the actual breast tissue. The majority of breast abnormalities are seen in this region, which contains almost all of the ducts and TDLUs. The US appearance of this zone depends on the physiologic state of the breast as well as the patient’s age. Within the ductal and lobular tissue, Cooper’s ligaments extend from front to back and further subdivide the mammary zone unpredictably. In addition, fibrous tissue and fat lie within the mammary zone.2
There are four main parenchymal patterns evident on ultrasound of the breast: fibrous, premenopausal, post-menopausal, and pregnant. The juvenile or fibrous breast contains little fat, and therefore the glandular region in this pattern is hyperechoic. The premenopausal breast is usually partially involuted, with the glandular region containing more subcutaneous and retromammary fat with interspersed fatty lobules. The fatty lobules may have a spherical appearance in one projection, and tubular appearance when the probe is turned 90 degrees. With pregnancy and lactation, there is an increase in glandular tissue that appears more hyperechoic, and the ductal structures are easily visualized. With this increase in glandular tissue comes compression of the subcutaneous and retromammary fat. The postmenopausal breast becomes fatty replaced, with the occasional presence of glandular elements.11
The nipple region contains dense connective tissue, which causes dramatic shadowing. This can make the resolution of structures immediately adjacent to the nipple difficult. The subareolar region is usually scanned at a tangential angle to avoid this shadowing.
The retromammary zone lies between the mammary zone and the pectoralis major muscle, and can be difficult to see on ultrasound. If seen, it usually appears as a narrow hypoechoic strip between the muscle and the glandular tissue. This zone contains mostly retromammary fat and some fibrous ligaments. Most pathologic processes that involve this zone do so secondarily, due to extension of malignancy or other processes from the mammary zone.2
When performing ultrasound on the breast, the premammary and retromammary fascia may be difficult to visualize. In older women, the breast has undergone fatty involution and the premammary fascia is not well seen, while in younger patients it can be dense (appearing as a well-defined thin line). The retromammary fascia is even more difficult to identify, and is often more readily seen on the lateral aspects of the breast where the breast is being pulled away from the chest wall.10
The chest wall is posterior to the retromammary zone. The most anterior structure seen in this region is the pectoralis major muscle, which appears as a hypo- or hyperechoic structure (depending on transducer angle) above the ribs. It has a striated appearance consistent with skeletal muscle. The pectoralis major muscle can serve as an orienting structure, since it is constant in location and relatively easy to identify. The intercostal and serratus anterior muscles are behind the pectoralis major muscle, followed by the ribs posteriorly. The ribs appear as hyperechoic crescents with posterior shadowing. The pleura and lung are seen deep to these structures, and the pleura can be seen moving with respiration.2,10,11
In describing a lesion on ultrasound, a standard or reference for echogenicity must be designated. Current practice is to use normal breast fat as the standard, since all women have fat in the subcutaneous zone regardless of the density of their breast tissue. Tissues that appear identical to the subcutaneous fat are considered isoechoic, tissues blacker than fat are hypoechoic, and those that are whiter than fat are hyperechoic. If a lesion lacks an internal echo, it is considered anechoic.12
Evaluating an entire duct is necessary because pathology can occur anywhere along its course. Generally, normal TDLUs that lie anterior to the main lobar ducts have longer extralobular terminal ducts than the posterior ones. This can result in the “taller than wide” appearance of cancers. The majority of TDLUs are in the mammary zone; however, they can extend into Cooper’s ligaments as well as into the premammary zone. TDLUs are normally 1–2 mm in diameter; however, they can vary greatly in size. Larger TDLUs are more easily seen sonographically than smaller TDLUs.5,11
SCANNING TECHNIQUE FOR OBTAINING THESE IMAGES
Breast ultrasound is best performed with high-resolution equipment, and is highly operator-dependent. High-frequency linear array transducers are required due to the wider near field, which is especially important for US-guided procedures. The 2011 standards from the ACR suggest transducer frequencies of 10 MHz and preferably higher. The frequency should be appropriate for the size and depth of the abnormal area, using the highest frequency capable of penetrating to the depth of the lesion of interest. The power, time-gain-compensation (TGC) curve, and focal zone settings should also be optimized. The power is kept as low as possible such that the beam just penetrates the chest wall. The TGC should be set to allow even penetration of the entire field. Power and gain should not be so high as to create artifactual echoes within a simple cyst, causing it to appear solid. They also should not be so low as to miss real echoes in a solid mass. The focal zone is electronically adjustable, and should be set at a lesion’s depth. Multiple focal zones are often needed to fully characterize a lesion.7,11
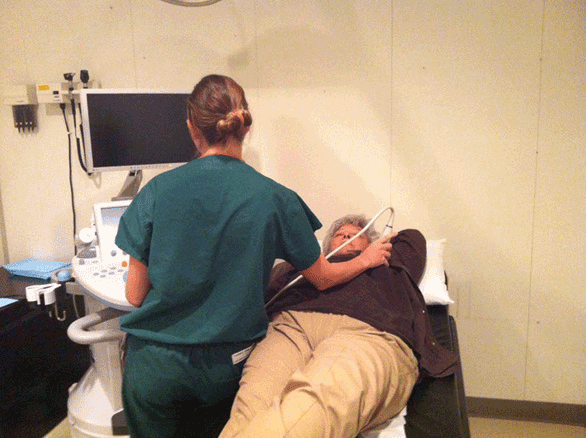
Proper positioning of the US machine, examiner, and patient is critical for a successful examination (Figure 5-2). The machine is usually placed to the patient’s right, and the (right-handed) examiner scans with the right hand and operates the machine with the left hand. For an image-guided procedure, the machine is placed on the opposite side of the patient than the side of interest. The ultrasound machine is kept parallel to the examining table, with the control panel directly in front of the examiner. The screen should be easily visualized by the examiner. The room should be warmed and the gel appropriately warmed to optimize patient comfort and facilitate the most successful examination.12 The patient should be asked if there is an area of palpable concern.
A generous amount of warm gel should be placed on the breast, with the transducer face maintained perpendicular to the skin. Gentle continuous pressure should be applied to maintain transducer-skin contact. This contact can be difficult to maintain in the region of the nipple-areola complex due to the wrinkling of the areola; however, copious gel can help. Application of even pressure with the transducer can decrease reflective and refractive attenuation. Transducer pressure can be increased for greater penetration of the tissue as necessary, particularly in the subareolar region.
The patient is positioned supine with a pillow behind the shoulder and the ipsilateral arm raised above the head, which flattens the breast and minimizes tissue depth. The patient can be moved to optimize imaging based on location of the lesion. For medial lesions, the patient is best positioned supine with the ipsilateral arm raised above the head. Lateral lesions are visualized with the patient posterior oblique, and the ipsilateral arm over the head. The superior aspect of a pendulous breast is best examined with the patient sitting, and the ipsilateral arm over the head. The examiner should make sure the patient is comfortable before beginning the examination. Also, it is important to ask the patient if they can feel an abnormality in a particular position.6,9,11
Numerous scanning methods are described in the literature. The American Society of Breast Surgeons recommends a standard scanning protocol including a radial scan, transverse sweep, and a tangential scan of the nipple. The footplate is oriented with the nipple on the left side of the viewing screen, along the radius of the breast in that particular region. This lines up the probe and image with the ductal anatomy of the breast, allowing visualization along the entire length of a ductal system. The radial scan is performed in a clockwise fashion, using the nipple as a pivot for the transducer. A rocking motion is used to better visualize a particular region. This standard approach allows relocalization in the future, if a lesion is visualized. Each quadrant is imaged from the nipple region to the periphery of the breast. The number of radii needed to examine a breast depends on the volume of breast tissue present in a particular patient, and usually ranges from 12 to 30. It is important to ensure adequate overlap of the scanning areas, particularly at 12, 3, 6, and 9 o’clock positions.
The transverse sweep or scan involves rescanning the entire breast from top to bottom, with the transducer oriented transversely. This can be started either medially or laterally, and provides a second look of the same tissue that was examined in a radial direction.9,11,12
The nipple-areola region is difficult to evaluate because scanning the nipple directly from above can cause a dense shadowing, which obscures underlying anatomy. The tissue behind the nipple is best evaluated by angling the probe tangentially. Applying pressure with the index finger of the opposite hand to the tissue on the opposite side of the nipple from the transducer improves visualization.2 Alternatively, the compression technique can also be used to visualize the nipple, by placing the opposite hand on the lateral aspect of the breast and pushing medially. This allows the transducer to move toward the nipple to obtain better visualization. This region should be scanned from both above and below the nipple, allowing the entire ductal system of interest to be visualized within the nipple and into the breast.11,12
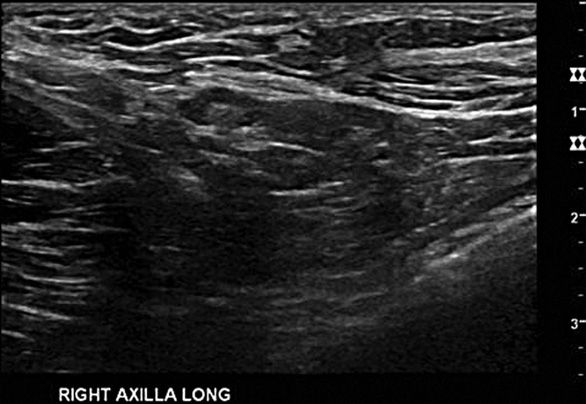
The axillary contents may be included in breast ultrasonography as well, and should be routinely evaluated in the setting of known malignancy. Scanning of the axilla is done in two planes. The axillary artery can be located by palpation, and is a useful guide for orienting the probe. Alternatively, the head of the humerus can be found with the probe and used as the superior most border of the axilla. The probe is then swept downwards through the axilla toward the tail of the breast. The longitudinal plane can be established by identifying the pectoralis major muscle and orienting the probe parallel to the muscle. Overlapping passes should be made in the axilla just as in the breast examination (Figure 5-3).9,12
Doppler examination can be utilized on the breast as well as the axilla, and may help distinguish between ducts and vessels within the breast.13 The axillary vessels can be visualized and their proximity to lesions of interest noted within the axilla. Normal lymph nodes are often not visualized, and enlarged lymph nodes should be considered pathologic. Pathologic nodes may be reactive or malignant.
Once an abnormality is identified, a targeted approach is used. The lesion should be evaluated in multiple planes by turning the transducer, and must be visualized in at least two planes to confirm that it is in fact abnormal. Images should be captured and measurements taken. Documentation of a lesion should be done in a standard format, including the following information (Table 5-3). Differential diagnosis of a solid mass includes the following (Table 5-4).