, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
Coronary artery anomalies represent a heterogeneous group of congenital disorders with different pathophysiology and clinical impact. By definition, they represent variants or deviations from the normal coronary artery anatomy [1].
Coronary artery anomalies represent a heterogeneous group of congenital disorders with different pathophysiology and clinical impact. By definition, they represent variants or deviations from the normal coronary artery anatomy [1].
16.1 Normal Anatomy of the Coronary Arteries
The aortic valve has three leaflets or cusps named the left, right, and noncoronary cusps. Just above the aortic valves there are three anatomic dilatations of the ascending aorta, known as the aortic sinuses of Valsalva. There are three sinuses of Valsalva which are named for the associated aortic valve cusp: left, right, and noncoronary. The left main coronary artery (LMCA) normally arises from the left sinus of Valsalva and the right coronary artery (RCA) arises from the right sinus of Valsalva. The noncoronary cusp is normally not associated with a coronary artery ostium.
The classic features of the normal coronary artery system include a right and left coronary circulation with separate ostia located at the aortic sinuses with 45–90° angles of origin from the aortic wall. The left coronary circulation has a common trunk called the LMCA that gives rise to all of the left coronary circulation branches. The proximal course of the LMCA coronary artery traverses the atrioventricular groove. There are branches to the ventricular myocardium and to the coronary arteries that terminate at the capillary bed. The coronary arteries are classified as “end circulation” since they represent the only source of blood supply to the myocardium [2].
16.1.1 Left Main Coronary Artery
The LMCA arises from the left aortic sinus of Valsalva. It bifurcates into the left anterior descending artery (LAD) (embedded in the anterior cardiac surface) and the left circumflex artery (LCx) (embedded in the posterior surface). Occasionally, the LMCA trifurcates into the LAD, the LCx, and a third vessel termed the ramus intermedius artery which arises between the takeoff of the LAD and LCx. The LAD and LCx arteries give off diagonal arteries and obtuse marginal arteries, respectively.
16.1.2 Right Coronary Artery
The right coronary artery (RCA) originates from the right aortic sinus of Valsalva and travels caudally through the right atrioventricular groove to reach the cardiac apex. The RCA gives off several branches, including the conus artery, sinus node artery, acute marginal artery, and posterior descending artery.
16.1.3 Epidemiology and Clinical Importance of Coronary Anomalies
The incidence of coronary artery anomalies is relatively rare and is estimated to affect approximately 1 % of the general population [1]. Yamanka and Hobs [3] reported a 1.3 % incidence of coronary anomalies in a series of 126,595 patients undergoing coronary angiography over a 28-year period. Although coronary artery anomalies are far less common than acquired coronary artery disease, they may have significant cardiovascular consequences, including myocardial ischemia, fatal arrhythmias, and sudden cardiac death [4]. Patients in whom the proximal segment of an aberrant vessel is compressed between the aorta and pulmonary artery have a high risk of exercise-related cardiac death [5].
Coronary artery anomalies are most often classified by their origin, course, termination, or aberrant intrinsic anatomy (Table 16.1). They can be both hemodynamically significant and insignificant. Hemodynamically significant anomalies are those that affect myocardial perfusion, leading to an increased risk of myocardial ischemia or sudden death. These anomalies potentially include (1) ectopic coronary origin of the RCA or the left coronary artery (LCA) from the pulmonary artery, (2) ectopic coronary origin from the opposite aortic sinus, (3) single coronary artery, (4) coronary artery fistulae, and (5) myocardial bridging [5].
Table 16.1
Coronary artery anomalies
I. Anomalies of origination and course |
Absent left main trunk (split origination of left coronary artery) |
Anomalous origin near aortic sinus of Valsalva |
Anomalous origin from opposite of noncoronary sinus |
Anomalous origin outside sinus of Valsalva |
Anomalous pulmonary origin |
Anomalous non-pulmonary origin |
Single coronary artery |
II. Anomalies of intrinsic coronary arterial anatomy |
Congenital ostial stenosis/atresia |
Coronary ectasia/aneurysm |
Coronary hypoplasia |
Absent coronary artery |
Intramural course (myocardial bridging) |
Coronary artery crossing |
Doubled (duplicated) coronary artery |
Anomalous origination of coronary artery branches |
III. Anomalies of termination |
Decreased number of arteriole/capillary ramifications |
Coronary artery fistula |
Extracardiac connections |
16.2 Anomalous Origin and Course of the Coronary Arteries
Excluding myocardial bridging, coronary artery anomalies of origin and course account for most coronary artery anomalies [5]. They represent up to 87 % of coronary anomalies diagnosed at invasive coronary angiography. These anomalies include (a) absent left main trunk, (b) anomalous origin near the sinus of Valsalva, (c) anomalous origin from the opposite or noncoronary cusp, (d) anomalous origin outside the sinus of Valsalva, and (e) single coronary artery.
16.2.1 Absent Left Main Trunk
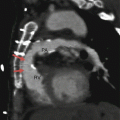
In this anomaly, there are three or more coronary ostia. The left main artery is absent and there are separate ostia for the LAD and LCx arteries with otherwise normal LAD and LCx artery anatomy (Fig. 16.1) [7]. In other words, the LAD and LCx arteries have separate ostia emanating from the aorta. This anomaly may cause technical difficulties when performing conventional selective angiography.


Fig. 16.1
Absent left main trunk. The separate ostia (black arrow in panel a and white arrow in panel b) of the left anterior descending artery (LAD) and circumflex artery (LCx) in panel (a), a 2D multiplanar reformat and panel (b), a 3D volume-rendered reconstruction. Ao aorta
16.2.2 Anomalous Coronary Artery Origin near the Proper Sinus of Valsalva
This group of anomalies includes (a) a high ostium and (b) commissural ostium. High takeoff or high ostium refers to the origin of either coronary artery ostium above the sinotubular junction (i.e., between its sinus of Valsalva and the tubular part of the ascending aorta). See Figs. 16.2 and 16.3. This anomaly involves the RCA more frequently than the left and rarely has clinical significance unless interventional procedures are performed at which time it may add difficulty to the procedure. Awareness of the abnormal anatomy is important in order to avoid accidental ligation, transection, or cross-clamping at the time of surgery as well as to simplify the cannulation of the artery during surgery or arteriography. The coronary artery origin is usually located a few millimeters above the sinotubular junction, but an origin >5 cm above the junction has been reported [8].



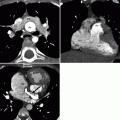
Fig. 16.2
The high takeoff of the left main coronary artery (LMCA) in a 2D multiplanar reconstruction (panel a) as well as in a 3D volume-rendering reconstruction (panel b). The ostium of the left main coronary artery (LMCA) is located above the sinotubular junction of aorta. LAD left anterior descending artery, LCx left circumflex artery, RCA right coronary artery, RI ramus intermedius artery, Ao aorta, LV left ventricle, RV right ventricle

Fig. 16.3
The high takeoff of the right coronary artery (RCA) in 2D multiplanar reconstruction (panel a) as well as in a 3D volume-rendering reconstruction (panel b) and a coronary artery tree format (panel c). The ostium of RCA is positioned above the sinotubular junction of aorta. LAD left anterior descending artery, LCx left circumflex artery, LMCA left main coronary artery
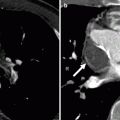
Commissural coronary artery ostium is an extremely rare variant in which a coronary artery originates near or behind the commissure between two aortic valve cusps (Fig. 16.4). The combination of a slit-like coronary ostium and intimal proliferation of the aortic valve leaflet can contribute to lumen obstruction. This anomaly has been rarely associated with sudden cardiac death [9].


Fig. 16.4
The commissural origin of left main coronary artery (LMCA). Note the presence of hypoplasia (small caliber) of the anomalous LMCA. The LMCA ostium is located at the commissure between left and noncoronary leaflets (arrow). R right coronary leaflet, RCA right coronary artery, L left coronary leaflet, N noncoronary leaflet
16.2.3 Anomalous Coronary Artery Origin from the Opposite or Noncoronary Sinus of Valsalva
Anomalous coronary artery origin from the opposite or noncoronary sinus of Valsalva represents the second most frequent reason for sudden cardiac death in the young (particularly during exercise) behind anomalous origin of the coronary artery near the sinus of Valsalva [10]. The diagnosis is challenging since patients have few prodromal symptoms and routine echocardiography is not sensitivity for detecting this abnormality [11–13]. This anomaly may be associated with sudden death, chest pain, arrhythmia, exercise-induced presyncope or syncope, left ventricular dysfunction, and findings of myocardial ischemia [14].
There are four anatomic patterns of anomalous origin of a coronary artery from the opposite or noncoronary sinus of Valsalva: (1) the LMCA arising from the right coronary sinus (Figs. 16.5 and 16.6), (2) the RCA arising from left coronary sinus (Fig. 16.7), (3) the LCx or the LAD arising from the right coronary sinus (Fig. 16.8), and (4) the LCA or the RCA arising from the noncoronary sinus (Fig. 16.9). There are also four different anatomical courses or distributions of the anomalous artery: (a) interarterial (between the aorta and pulmonary trunk, Fig. 16.7), (b) retroaortic (Figs. 16.5 and 16.6), (c) prepulmonic, and (d) septal (also known as subpulmonic, Fig. 16.6). The precise path taken by the artery is of clinical importance. The interarterial course (previously called a malignant course) is associated with a high risk of sudden cardiac death, while the retroaortic, prepulmonic, and septal courses are considered low-risk anomalies.






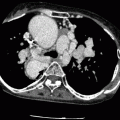
Fig. 16.5
The abnormal origin of left main coronary artery (LMCA) from the right coronary sinus together with the right coronary artery (RCA) in a maximum intensity projection (panel a), a 3D volume-rendered image (panel b) and a coronary artery tree format (panel c). The LMCA follows the retroaortic course (seen best in panels a and b) and bifurcates subsequently into the left anterior descending artery (LAD) and circumflex artery (LCx). Note the normal width of LMCA ostium and the acute angle (arrowhead) just after its origin. The arrow in panel (a) points to the RCA ostium which is moving out of plane. Ao aorta

Fig. 16.6
The abnormal origin of left main coronary artery (LMCA) from the right coronary sinus together with the right coronary artery (RCA) in 2D multiplanar reformat (panels a and b) and 3D volume-rendered reconstruction (panel c). The LMCA follows the subpulmonary course (single arrow in panel b and multiple arrows in panel c). Note the hypoplasia of the anomalous artery (small caliber) and acute angle (arrowhead in panel a) at its origin. PA pulmonary artery

Fig. 16.7
The abnormal origin of right coronary artery (RCA) from the left coronary sinus together with the left main coronary artery (LMCA) in 2D multiplanar reformat (panels a and b) and 3D volume-rendered reconstruction (panel c). The RCA follows the interarterial course (between the pulmonary artery and aorta). Note the hypoplasia (small caliber), acute angle takeoff, diagonal course between the great arteries and slit-like origin all of which define it as intramural (arrowhead in panel a). This anomaly takes intramural course as identified by the acute angle takeoff and the slit-like origin and diagonal course as it travels between the aorta and the pulmonary artery. LAD left anterior descending artery, PA pulmonary artery, Ao aorta

Fig. 16.8
The abnormal origin of left circumflex artery (LCx) from the right coronary sinus together with the right coronary artery (RCA) in a multiplanar reformat (panels a and b) and 3D volume-rendered reconstruction (panel c) and a coronary tree format (panel d). The LCx follows the retroaortic course. Note the normal position of the left anterior descending artery (LAD). R right aortic sinus of Valsalva, L left aortic sinus of Valsalva, N noncoronary aortic sinus of Valsalva

Fig. 16.9
The abnormal origin of the left main coronary artery (LMCA) from the noncoronary sinus. Panel (a) is a multiplanar reformat and panel (b) is a 3D volume-rendered reconstruction. The right coronary artery (RCA) arises normally from the right coronary sinus. LAD left anterior descending artery, LCx left circumflex artery, R right coronary sinus, N noncoronary sinus, L left coronary sinus
High-risk anatomy (i.e., high risk for sudden cardiac death) can be associated with other abnormalities including a narrowed slit-like orifice of the coronary ostium, acute angle takeoff of the ostium, and an intramural course. An intramural course is characterized by a coronary artery whose origin travels diagonally through the wall of the aorta before exiting the aortic lumen as opposed to taking a perpendicular course directly through the aortic wall as it exits the aorta. This is identified by the acute angle takeoff, the slit-like origin, and the diagonal course between the great vessels [15]. To date, no single anatomical abnormality has been identified as the definitive cause of ischemia and sudden cardiac death [16].
The LMCA arising from the right coronary sinus of Valsalva is reported to occur in 0.017 % of patients undergoing coronary angiography (Figs. 16.5 and 16.6) [3]. It is associated with a high incidence of sudden cardiac death which is related to its frequently occurring interarterial course [17]. The interarterial course is associated with coronary arterial compression (worse in systole) which decreases coronary blood flow and may result in myocardial infarction. Anomalous coronary arterial flow can be further compromised during exercise due to aortic dilatation. A narrow slit-like orifice and an acute angle takeoff of the coronary ostium with a tangential proximal course of the ectopic coronary artery are also common pathologic findings and can contribute to sudden cardiac death. Surgical coronary revascularization is recommended in all patients with an anomalous, interarterial LMCA [14].
Less commonly, an anomalous LMCA may also take a retroaortic, prepulmonic, or septal (subpulmonic) course.
The RCA arising from the left coronary sinus Valsalva has been reported in approximately 0.2 % of patients scheduled for coronary angiography [3] (six times higher incidence than the LMCA arising from the right coronary sinus) (Fig. 16.7). It is associated with a lower risk of sudden cardiac death than LMCA arising from the right coronary sinus [12, 15, 18]. The most common course of an anomalous RCA arising from the left sinus of Valsalva is interarterial so this variant can be associated with sudden cardiac death [5].
Within the LCx or LAD arising from the right coronary sinus class of coronary anomalies, the LCx artery more frequently arises from the right coronary sinus than does the LAD artery (Fig. 16.8) [3, 19]. The vast majority of the time, the anomalous LCx artery passes behind the aortic root (i.e., retroaortic course) and has a good prognosis. It has not been associated with sudden death [20]. The LAD arising from the right coronary sinus is a very rare malformation. Rarely seen in patients with otherwise normal hearts, it has been associated with tetralogy of Fallot, double outlet right ventricle, and transposition of the great arteries. It may take either an interarterial or an intraseptal or prepulmonic course. The interarterial course portends a serious outcome, while the other intraseptal and prepulmonary routes are considered low-risk anomalies.
16.2.4 Anomalous Origin of the Coronary Artery Outside the Aortic Sinuses of Valsalva
Anomalous coronary arteries originating outside aortic sinuses of Valsalva may have a pulmonary origin or a non-pulmonary origin.
There are five anatomic patterns of anomalous pulmonary origin of coronary artery: (1) anomalous LMCA (LCA, left coronary artery) from the pulmonary artery (also known as ALCAPA or Bland–White–Garland syndrome), (2) anomalous RCA from the pulmonary artery, (3) anomalous LCx artery from the pulmonary artery, (4) anomalous LAD artery from the pulmonary artery, and (5) anomalous RCA and LCA from the pulmonary artery [6]. Of those anomalies, ALCAPA is most common, while the other variants are extremely rare.
ALCAPA (Bland–White–Garland syndrome, Fig. 16.10) has an incidence of 1 in 300,000 live births [14]. This anomaly results in a coronary steal phenomenon caused by the flow of blood from the higher pressure coronary arterial system to the lower pressure pulmonary arteries and results in impaired myocardial perfusion.




Fig. 16.10
Anomalous pulmonary origin of left main coronary artery (LMCA) from the main pulmonary artery (MPA) also known as ALCAPA or Bland–White–Garland syndrome. Panels (a) and (b) are multiplanar reformats demonstrating the takeoff of the LMCA from the MPA. Panels (c) and (d) illustrate the very large and tortuous right coronary artery (RCA) which provides oxygenated myocardial blood supply for the whole myocardium via an extensive collateral circulation to the branches of the left coronary circulation and seen best in panel (c and d) and shown by the arrows. Note the flow of blood from the left coronary artery to the MPA. Aoasc ascending aorta, LA left atrium, LAA left atrial appendage, LAD left anterior descending artery, LCx left circumflex artery, LV left ventricle, RA right atrium, RV right ventricle, SVC superior vena cava
Based on age at presentation and survival patterns, ALCAPA has been classified as infantile types or adult types [23]. The infantile form presents days to weeks after birth with severe left ventricular dysfunction and mitral regurgitation due to an inadequate intercoronary collateral circulation. The prognosis is poor in the absence of early surgical intervention and approximately 90 % of untreated infants die in the first weeks to months of life. Very few patients survive to adulthood [24].
In the adult type, the development of extensive intercoronary collaterals, which feed the LCA territory, as well as a restrictive opening between the LCA and the pulmonary trunk, lead to near-normal left coronary perfusion and longer survival [25]. These patients may remain asymptomatic until adulthood despite silent, recurrent ischemia. In adults, clinical manifestations of ALCAPA include left ventricular dysfunction, mitral regurgitation, exercise-induced myocardial ischemia, myocardial infarction, heart failure, cardiac arrhythmia, and sudden cardiac death.
The anomalous LCA may arise from the pulmonary artery at or above the level of the pulmonary sinuses (high takeoff) or very close to a valvular commissure. At the pulmonary sinus level, the anomalous ostium most commonly originates from the left posterior pulmonary sinus (so-called right-handed sinus). Less commonly, it arises from the right posterior (left-handed) sinus or from the anterior (nonfacing) pulmonary sinus [26, 27].
Once the diagnosis of ALCAPA is established, prompt surgical reconstruction is indicated to restore a normal dual coronary artery, which usually results in near-total myocardial recovery [88]. In infantile-type ALCAPA, the surgical reconstruction strategies include direct reimplantation of the anomalous LCA into the aorta, creation of an intrapulmonary baffle from the left coronary ostia to the aorta (Takeuchi procedure, Fig. 16.11), or coronary bypass grafting (Fig. 16.12) [25]. Since in most cases the anomalous ostium arises from the left posterior sinus, aortic reimplantation is usually technically easy and allows full anatomical correction. In adult-type ALCAPA, ligation of the LCA from the pulmonary artery, combined with coronary artery bypass grafting utilizing the internal mammary artery or a saphenous vein, is preferable [28]. If coronary reimplantation is not feasible due to an unfavorable coronary anatomy and or a short length of the coronary artery, the Takeuchi procedure may restore dual coronary artery supply [88].



Fig. 16.11
Takeuchi procedure in Bland–White–Garland syndrome (ALCAPA): Panels (a) and (b) are multiplanar reformats demonstrating the baffle from the main pulmonary artery (MPA) wall making an intrapulmonary tunnel (arrows) between the left coronary artery origin off of the MPA and the surgically created aortopulmonary window (creating a “pseudo left main coronary artery”). Panels (a) and (c) demonstrate the narrowed orifice of the surgically created intrapulmonary arterial baffle at aortic wall origin (arrowhead) with dilatation of the baffle after the stenosis (seen best in panel c). Aoasc ascending aorta, Aodesc descending aorta, PA pulmonary artery, LPA left pulmonary artery, RPA right pulmonary artery, RV right ventricle

Fig. 16.12
Coronary artery bypass grafting and ligation of the native left main coronary artery in a patient with ALCAPA (Bland–White–Garland syndrome): Panels (a), (b) are multiplanar reformats while panel (c) is a 3D volume-rendered image. Note the origin of the wide caliber of the right coronary artery (RCA) and the presence of the calcified venous graft (arrows) between the ascending aorta (Aoasc) and the left descending coronary artery (LAD). Aodesc descending aorta, LA left atrium, LAA left atrial appendage, LCx left circumflex artery, LPA left pulmonary artery, RPA, right pulmonary artery
Adults after surgical repair should be evaluated every 3–5 years to assess the adequacy of repair and to exclude ongoing or recurrent myocardial ischemia [14]. Potential post-repair complications may include coronary artery and graft stenosis as well as aortic and mitral valve insufficiency. After the Takeuchi procedure, supravalvular pulmonary stenosis and baffle leaks or baffle stenosis may develop [29–31].
In the anomalous non-pulmonary origin variant, the anomalous coronary artery may arise from the right or left ventricle, aortic arch, ascending or descending aorta, as well as from the carotid, subclavian, innominate, internal thoracic, or bronchial arteries [6]. These origins are extremely rare and the clinical findings as well as the age at diagnosis are variable.
16.2.5 Single Coronary Artery
The single artery system is a rare abnormality in which only one coronary artery arises from a solitary ostium [32]. The incidence of this anomaly in series of patients undergoing invasive coronary angiography ranges from 0.008 to 0.066 % and accounts for 1.1–8.8 % of all coronary anomalies [33]. A single coronary artery may be an isolated finding or it may be associated with other congenital heart diseases including transposition of the great arteries, tetralogy of Fallot, truncus arteriosus, and coronary artery fistulas [26, 32].
Three different types of single coronary artery have been described: (1) single coronary artery following the course of either a normal RCA or LCA, (2) single coronary artery giving rise to the left main and RCA, and (3) single coronary artery having an anomalous course that does not meet criteria for type 1 or type 2 [34].
16.3 Anomalies of Intrinsic Coronary Arterial Anatomy
Anomalies of intrinsic coronary arterial anatomy represent a heterogeneous group of abnormalities, including coronary ostial atresia and coronary ostial stenosis, coronary ectasia and coronary aneurysm, absent coronary artery, coronary artery hypoplasia, muscular bridging, coronary crossing, double coronaries, and anomalous origin of coronary artery branches.
16.3.1 Coronary Artery Ostial Atresia or Stenosis
Coronary ostial atresia refers to complete atresia of the coronary ostium. There are two morphologic variants: (1) atresia of the LMCA (commonest form) and (2) atresia of the RCA [35]. In atresia of the left coronary ostium, the entire coronary arterial supply is from the RCA and its branches. The LAD and LCx artery are seen in their normal respective locations, but they join together and terminate at a proximal blind end, receiving blood only from RCA collaterals [36, 37]. The collateral circulation from the right to the left coronary system is usually inadequate, and thus, most affected patients develop early myocardial ischemia and die in infancy or childhood. However, asymptomatic survival until adulthood has been reported and cases may be discovered incidentally at angiography [38].
This anomaly can also be associated with findings of myocardial ischemia or infarction, heart failure, syncope, and sudden cardiac death [36]. Surgical reconstruction of a two coronary artery system by a coronary artery bypass graft procedure is the preferred surgery [36].
In congenital right coronary ostial atresia, there is atresia of the right coronary ostium. In this rare condition, the RCA is supplied via collaterals from the LCA [39]. Surgical repair entails restoration of a dual coronary artery supply [39].
Congenital ostial stenosis is a multifactorial condition that may be caused by an acute angle takeoff of the coronary artery, a slit-like orifice, intimal proliferation of an aortic valve leaflet, fusion of the aortic leaflets, and intramural course of the coronary artery [71, 77]. Coronary ostial stenosis has been associated with sudden cardiac death [77, 80].
16.3.2 Coronary Artery Ectasia or Aneurysm
Coronary artery ectasia or aneurysm is defined as a dilated coronary artery with a luminal diameter exceeding 1.5 times the diameter of the adjacent normal coronary segment or the largest diameter of the coronary artery (Fig. 16.13) [40–42]. The dilatation is commonly diffuse and can be either fusiform or saccular in shape [44]. A giant coronary aneurysm is usually defined as a coronary artery with a maximal diameter exceeding 20 mm, although other cutoff values (50–150 mm) have also been described [41, 43].