and Ashutosh Dash2
(1)
Nuclear Security and Isotope Division, Oak Ridge National Laboratory, OAK RIDGE, USA
(2)
Isotope Production and Applications Division, Bhabha Atomic Research Centre, Mumbai, India
1.1 Introduction: Use of Radioisotopes in Nuclear Medicine
As a foundation for a discussion of the development and use of therapeutic radiopharmaceuticals, it is first necessary to provide an introduction to the field of nuclear medicine and how radioisotopes are used in this board-certified clinical specialty for both diagnostic and therapeutic applications. In the field of nuclear medicine, unsealed radioactive agents known as radiopharmaceuticals are administered generally intravenously for either diagnostic or therapeutic applications (McCready 2000; Ercan and Caglar 2000). These specialists and their staff are specially trained in the safe handling, storage, and disposal of radioactive materials. Special licensing is required, radiopharmaceuticals must be approved by regulatory bodies for use, and certified radiopharmacists are required for the formulation and dispensing of these radioactive substances. Diagnostic applications in nuclear medicine use low activity tracer levels of generally gamma- or positron-emitting radioisotopes which are generally produced in nuclear reactors and accelerators (Chap. 5). In contrast, therapeutic applications utilize particle-emitting radionuclides for induction of radiotoxicity to kill cells in the targeted tissue. The substrate or targeting moiety (vector) to which the radionuclide is chemically attached is designed to favor the accumulation of the administered radiopharmaceutical at the targeted cell, tissue, or organ. The radiation emitted from the accumulated radioactivity is then detected by external measuring devices such as a gamma camera or positron emission tomographic camera to reconstruct images for diagnostic purposes. For therapeutic applications in nuclear medicine, particles emitted from radioactive decay of selected radioisotopes deliver cytotoxic levels of radiation to the target site. After site-specific accumulation of the radiopharmaceutical to the target site, cytotoxic ionizing radiation is delivered to induce un-repairable double-strand DNA breaks which result in subsequent cell death (Hoefnagel 1998; Aerts et al. 2014; Wheldon 1994). It should also be noted that in the form of sealed radioactive sources, therapeutic radioisotopes are also used in other clinical specialties, most notably in brachytherapy practiced in radiation oncology. In these applications, permanently often reusable sealed radioactive sources are introduced adjacent to the target tissue for limited time periods and then removed, or permanently implanted, such as well-established methods for treatment of prostate cancer (Connell and Hellman 2009; Gerber and Chan 2008). This book focuses on the description of unsealed radioactive materials which are used for nuclear medicine therapy.
1.2 Key Examples of Nuclear Medicine
1.2.1 Nuclear Medicine Imaging
For imaging tissue anatomy, function, and metabolism, radioisotopes which decay by emission of gamma photons, X-rays, and positrons (β+) are targeted to specific organs and disease entities (James and Gambhir 2012; Zanzonico 2012). The radiopharmaceutical agents are generally administered intravenously and for some applications either orally or by inhalation, with the radioactive agent then localizing in the targeted specific organ or tissue. The emission of radiation from the localized radioactive agent is then detected by scintillation cameras and other instruments and the detector data is then processed in the computer into either two-dimensional (planar imaging) or three-dimensional (tomographic imaging) images of the radiopharmaceutical distribution. These data can also be used for several important applications on organ function to quantitate time–activity curves or the uptake and release kinetics of radioactivity over time. Images obtained from stationary camera devices are referred as scintigraphs, while the use of a linear moving or rotating camera system in two dimensions is called a scan. The use of the latter tomographic technology is currently most widely used for many applications, where the gamma camera is rotated around the patient to obtain three-dimensional images (Kjaer 2006). The development and clinical applications of radioisotopes and radiopharmaceuticals in nuclear medicine are widely described in the literature (Bhattacharyya and Dixit 2011; Britton 1997; Ercan and Caglar 2000; Hoefnagel 1991; Leeds 1990; Penner et al. 2009; Volkert and Hoffman 1999).
Modern gamma cameras provide tomographic images, and this technology is commonly referred to as single-photon emission computed tomography (SPECT). Another major nuclear medicine imaging modality is positron emission tomography (PET) which provides very high-resolution images and detects the coincidence photon events which occur after positron emission of radioisotopes such as carbon-11 (11C) and fluorine-18 (18F). Nuclear medicine imaging is thus unique, because it provides information about both structural and functional changes involved in a disease process and offers unique opportunities and can determine the presence of metabolic and functional abnormalities based on biological changes mostly at the cellular level rather than changes in anatomy, which are often only detected at later stages of the disease. Traditional radiology-based imaging technologies such as X-ray computed tomography (CT), ultrasound (US), and magnetic resonance imaging (MRI) are almost used exclusively for evaluation of anatomy. Nuclear medicine imaging using planar scanning, SPECT, and PET, however, can thus often reveal metabolic and physiological abnormalities generally not detected by static forms of anatomic imaging such as CT and US, although some unique physiologic applications are possible with the MRI modality. Important instrumental developments have progressed over the last two decades, however, by the combination of traditional anatomically based imaging modalities and radioisotope-based technologies. Current state-of-the-art imaging instruments are thus now widely used which simultaneously utilize both PET and SPECT in conjunction with MRI and CT in hybrid imaging instruments for accurate assessment of radiopharmaceutical targeting data with anatomical images (i.e., PET-CT, PET-MRI, SPECT-CT).
1.2.2 Molecular Imaging
Molecular imaging is a special nuclear medicine application where engineered radiopharmaceutical agents are specifically targeted for the detection and evaluation of functional changes at a specific cellular level, which allows monitoring of body function to measure specific chemical and biological processes (Bentzen and Gregoire 2011; Garden et al. 1989; Howard et al. 2015; Hunter and Eisbruch 2011; Maletz et al. 2012; Schlegel 2010; Simpson et al. 2009; Wolbarst and Hendee 2006). Molecular imaging can often uniquely identify disease processes at very early stages before clinical symptoms are observed. Several examples include the use of radiolabeled peptides to detect tumors. Nuclear medicine imaging thus plays a critical role at every phase of disease assessment, which includes diagnosis and staging, treatment planning, monitoring response to therapy, and monitoring recurrence and residual disease. In cancer management these technologies can detect disease conditions which may often be occult and be undetected by other imaging modalities. Imaging can also assess the severity of disease, including the degree of spread throughout the body. Although initial cancer staging has been historically accomplished by various diagnostic techniques such as CT and MRI, the relatively recent broad availability of SPECT and PET nuclear imaging technologies has resulted in new opportunities in cancer management, for example, for staging, upstaging, or downstaging specific cancer entities. In planning cancer treatment, nuclear medicine imaging often provides important information for selecting the most effective therapy based on the unique biologic characteristics of a particular patient and the molecular properties of the tumor. This “personalized” approach for patient management is important for evaluation of response to ongoing therapy, and such functional imaging technologies offer advantages not available through anatomic imaging alone. This critical advantage can allow midcourse alteration of treatment, if necessary, as opposed to the presentation of structural changes revealed by other imaging modalities. Nuclear imaging is also highly useful for detection of residual disease or surveillance for recurrence (Akkas et al. 2014; de Haas et al. 2012; Kostakoglu et al. 2013; Kurdziel et al. 2008). The key components which comprise the development of molecular imaging (MI) agents are depicted in Fig. 1.1.
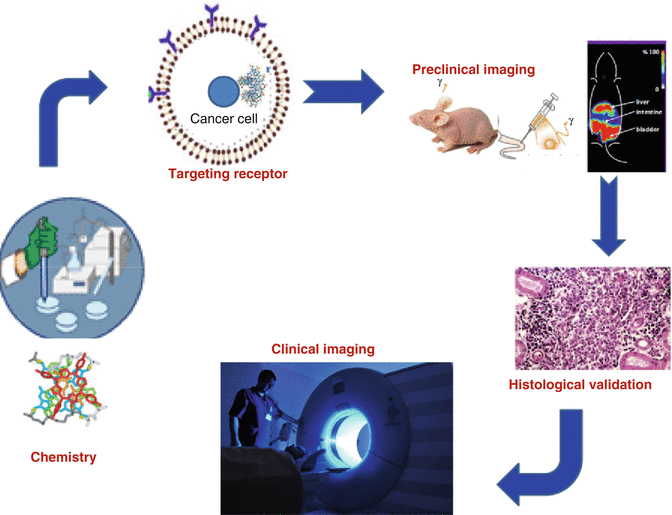

Fig. 1.1
The cycle of the development of molecular imaging agents
1.2.3 In Vivo Function Tests
In this process a radiopharmaceutical is administered, and the adsorption, distribution, metabolism, and excretion (ADME) properties are documented (Dalvie 2000; Giron et al. 2008). Functional processes that can be assessed include tissue blood flow and metabolism, protein–protein interactions, expression of cell receptors in normal and abnormal cells, cell–cell interactions, neurotransmitter activity, cell trafficking and homing, tissue invasion, and programmed cell death. By providing information on these processes, nuclear medicine imaging offers a broad array of tools for probing normal and disease-related states of tissue function and response to treatment. The time–activity curves of the organs of interest are obtained which reflect organ function. In vivo quantification of radiopharmaceuticals has great potential as a tool in assessing the function of an organ or organ systems. Key examples include kidney function tests, neurological disorders, cardiovascular disease, bile flow, lymphatic drainage, thyroid structure and function, small-bowel transit gastric emptying assessment, acute GI bleeding, hepatic hemangiomas, reticuloendothelial function renal, artery stenosis, and inflammatory bowel disease (Alazraki 1993; Bomanji and Siraj 1995; Eberlein et al. 2011; El-Maghraby et al. 2006; Ganz and Serafini 1989; Messa et al. 1995; Notghi and Harding 1995; Prvulovich and Bomanji 1998; Ross 1991).
1.2.4 Nuclear Medicine Therapy
The primary focus of this book is the use of unsealed radioactive sources for nuclear medicine therapy, which is commonly referred to as radionuclide therapy (RNT). In this therapeutic modality, high activity levels of generally particle-emitting radioisotopes attached to tissue-targeting agents are administered to deliver high radiation doses to targeted tissues. Principal established clinical applications include cancer therapy (Chaps. 9 and 10), treatment of metastatic bone pain (Chap. 12), and treatment of inflammatory processes such as rheumatoid arthritis (Chap. 14). Key examples of developing technologies using therapeutic radiopharmaceuticals include treatment of nonmelanoma skin cancer in delicate anatomical areas (Chap. 13) and therapy of hyperplasia which often occurs after arterial angioplasty (Chap. 15). These applications and the radiopharmaceuticals which are employed are discussed in subsequent chapters. A large variety of disease-targeting radiopharmaceuticals to which are attached radionuclides which emit alpha (α), beta (β−), or Auger (AE)/conversion (CE) electron particulate radiation are used for RNT. Unlike conventional external beam therapy—under the purview of radiation oncology—RNT is practiced in the nuclear medicine arena and targets diseases at the cellular rather than on a gross anatomical level (Cuaron et al. 2009: Dash et al. 2013; Eary 1991; Gabriel 2012; Srivastava and Dadachova 2001; Yeong et al. 2014). This concept is a blend of a tracer moiety that mediates a site-specific accumulation followed by induction of cytotoxicity with the short-range biological effectiveness of the particulate radiation. The proximal contact between the radionuclide and the cells targeted for destruction enables the absorbed radiation to be concentrated at the target site with the minimal injury to adjacent healthy tissue.
1.3 Radiopharmaceuticals
In contrast to the common use of nonradioactive therapeutic routine pharmaceuticals, radiopharmaceuticals are generally administered at sub-pharmacologic dose in very high specific activity (radioactivity/unit mass). Radiopharmaceuticals can consist in some instances of a radionuclide in ionic form—such as iodine-131 (131I) for treatment of thyroid cancer or strontium-89 (89Sr) for bone pain palliation (Chap. 12)—but these agents are generally represented by pharmaceutical targeting agents to which the radioisotope is chemically attached. These carrier molecules are represented by a broad range of molecules, which include chelating agents, small molecules, drugs, peptides, proteins, or particles. Similar to conventional pharmaceuticals, the radiopharmaceutical targeting agents are administered by oral, intra-arterial, intravenous, intratumoral, intra-portal, and intracavity routes. The administered radiopharmaceuticals accumulate in the organ or tissue of interest through a variety of well-established known biological mechanisms, for which these agents are designed and developed. The radionuclides attached to the radiopharmaceuticals provide the radiation component (radioactivity), while the carrier molecule targets specifically diseased tissues or cells. These radiopharmaceutical carrier molecules to which radionuclides are attached are often called vectors and often consist of small organic molecules such as a drug, carbohydrate, lipid, nucleic acid, peptide, fragment of antibody, or even very large whole antibodies (Cutler et al. 2013; Dash et al. 2013). The synthetic chemical and biological issues associated for vector selection, development, and preparation are challenging and key factors to provide these agents for preclinical evaluation with a goal of eventual clinical application and are based on the ability of radiopharmaceuticals to accumulate selectively on/or/in a cell, tissue, or organ.
The activity doses of radiopharmaceutical used for diagnostic imaging applications vary depending on the extent of accumulation at the target site, the residence time, release kinetics, type of investigation being conducted, and the imaging technology which is employed. The goal is to limit the activity levels which are required for imaging to minimize the radiation dose to no targeted tissues. Often millicurie (mCi) levels of radioactivity are adequate for diagnostic studies, whereas several hundreds of mCi of activity are often required for therapeutic applications. Radiopharmaceuticals are formulated in various chemical and physical forms and require production and dispensing under good manufacturing conditions (GMP). There are several hundred radiotracers which have been developed over the last decades which have potential radiopharmaceutical use in humans. The radioisotopes used for both diagnostic and therapeutic applications are generally produced in research reactors and accelerator facilities or are available from radionuclide generator systems, as described in Chaps. 5, 6, 7, and 8. Although some imaging and therapeutic agents are produced or dispensed in-house in a hospital-based radiopharmacy under carefully controlled conditions, most radiopharmaceutical agents are delivered for clinical use in a ready-to-use form by commercial manufacturers or from a central radiopharmacy.
Radiopharmaceuticals are available in a wide variety of chemical and physical forms which include radionuclides in inorganic forms, such as Na131I (thyroid therapy), 90SrCl2 (bone pain palliation), and Na99mTcO4 (thyroid imaging). Radionuclides are generally attached by complexation to suitable chelating groups, and these entities are then used for imaging or therapy. Key diagnostic examples include 99mTc-MDP (methylene diphosphonate) for bone imaging and 99mTc-MIBI (methoxy isobutyl isonitrile), which is widely used for assessment of regional myocardial perfusion. In addition, radionuclides can be complexed to a suitable chelating agent which is attached to the targeting vector, and key examples in this class include 99mTc-Hynic-TOC (a somatostatin analog peptide) used for tumor detection and therapy management evaluation. In addition, another important strategy is covalent linkage of radionuclides to drug molecule, and examples include 131I-MIBG (MIBG: metaiodobenzylguanidine) for the treatment of adrenal-based tumors, primarily in children. Finally, therapeutic radionuclides can also be attached by chemical chelation to a carrier vector such as 99mTc-UBI (UBI, ubiquicidin). Key examples of different types of widely used radiopharmaceuticals and their uses are provided in Table 1.1.
Table 1.1
Examples of key diagnostic and therapeutic radionuclides and radiopharmaceutical agents used in nuclear medicine applications
Radionuclide | Agent | Chemical form | Application |
|---|---|---|---|
Diagnostic radiopharmaceuticals | |||
18F | 18F[FDG] | Vector-link | Imaging cell proliferation |
123I | 123I-MIBG | Vector-link | Imaging medullary carcinoma |
99mTc | Na99mTcO4 | Ionic | Thyroid scanning |
99mTc | 99mTc-MDP | CA | Bone imaging |
99mTc | 99mTc-DTPA | CA | Renal agent for estimation of glomerular filtration rate (GFR) estimation |
99mTc | 99mTc-MIBI | CA | Cardiac imaging |
99mTc | 99mTc-ECD | CA | Brain imaging |
99mTc | 99mTc-hynic-TOC | Vector-CA | Imaging neuroendocrine tumors |
99mTc | 99mTc-hynic-RGD | Vector-CA | Imaging neo-angiogenesis |
99mTc | 99mTc-UBI | Imaging infection | |
Therapeutic radiopharmaceuticals | |||
131I | Na131I | Ionic | Thyroid scanning, treatment of hyperthyroidism and thyroid cancer |
131I | 131I-MIBG | Treatment of medullary carcinoma | |
177Lu | 177Lu-DOTATATE | Vector-CA | Treatment of neuroendocrine tumors |
89Sr | 89SrCl2 | Ionic | Bone pain palliation |
90Y | Zevalin® | Vector-CA | Treatment of non-Hodgkin’s lymphoma |
The development and use of radiopharmaceuticals is truly a multidisciplinary process. Key strategies for radiopharmaceutical development include a number of physiological issues which are required to insure that the expected targeting and kinetics are optimized. Radiopharmaceuticals should exhibit rapid blood clearance and, when appropriate, possess high membrane permeability to facilitate cellular accumulation. The agents should also exhibit slow metabolism before delivery and after accumulation at the target site and minimal accumulation in nontarget organs. In addition, limited transport and biochemical transformation should occur to facilitate kinetic modeling of the tracer. These data are generated from time–activity curves and serial imaging and allow an estimation of radiation dose to both target and nontarget organs. Radiopharmaceutical development encompasses a variety of disciplines and capabilities including radionuclide production (Chaps. 5, 6, 7, and 8) with subsequent radiochemical processing, purification, and analysis to provide the radionuclides with requisite purity. Other key capabilities and resources include the organic synthesis of chelating agents or vectors for radiolabeling with the appropriate properties, and the development of radiolabeling methods ensures high radiochemical yields. Rapid and efficient purification procedures are required to obtain high radiochemical purity of the final products. From a pharmaceutical perspective, reliable technologies are required for the routine production of sterile, pyrogen-free products with minimum inconvenience achieved either through kit formulation procedure or through the use of automated synthesis modules. Finally, simple quality control procedures are required to ensure the purity and assurance for human use.
1.3.1 Diagnostic Radiopharmaceuticals
The use of radiopharmaceuticals for imaging organ function and disease involvement is the unique capability of nuclear medicine. Radiopharmaceuticals used in diagnostic nuclear medicine procedures generally emit either gamma radiation or positrons, and the half-lives of radionuclides for imaging applications generally span from minutes to several hours. Although it is not a goal of this book to discuss these diagnostic agents in detail, Table 1.2 provides a summary of commonly used radionuclides for diagnostic radiopharmaceuticals.
Table 1.2
Key examples of radionuclides used for diagnosis in nuclear medicine
Radionuclide | Half-life | Emission |
|---|---|---|
15O | 2.1 min | Positron |
11C | 20.4 min | Positron |
68Ga | 60 min | Positron |
18F | 109.8 min | Positron |
99mTc | 6 h | Gamma |
111In | 2.8 days | Gamma |
123I | 13.2 h | Gamma |
1.3.2 Nuclear Medicine Imaging
Radiopharmaceuticals are administered to patients and then redistributed by well-established physiological principles which depend on specific properties of the targeting agent. All radiopharmaceuticals are carefully designed and have well-defined properties which govern adsorption, distribution, metabolism, and excretion (ADME). Nuclear medicine imaging is conducted at defined time points which are evolved as the imaging protocols (Zanzonico 2012). Usually imaging commences after clearance of blood pool activity. The evolution of nuclear medicine imaging has taken many twists and turns as both equipment technology and new radiopharmaceuticals have been developed over the past decade. The commonly used planar, SPECT, and PET imaging techniques are discussed in more detail below.
1.3.2.1 Planar Imaging
A schematic representation of planar and SPECT imaging principles is illustrated in Fig. 1.2. Detection and subsequent image reconstruction of gamma rays emitted by radiopharmaceuticals is accomplished by scintillation cameras also referred to as Anger or gamma cameras (Fig. 1.2a). The gamma rays emitted by the radionuclide are detected by the scintillation crystal, which is usually thallium-activated sodium iodide (Anger 1958; Erickson 1992; Tapscott 1998; Pexman 1973; Telander and Loken 1967). The ionizations produced within the sodium iodide crystals emit secondary radiations which are converted to light photons via sodium iodide scintillation detectors, and photodiodes then convert the secondary radiation to light photons which are amplified by a series of photomultiplier tubes before measurement as a current pulse. Reconstruction is accomplished using computer software which converts the current detected at different points corresponding to the crystal to images. These images are traditionally called scintigraphs and are displayed as two-dimensional views of the targeted site region of interest. Such 2D images, known as planar scintigrams, are often of poor quality due to the superposition of nontarget activity from the 3D body which restricts the measurement of organ function and prohibits accurate quantification of that function. However, in some cases, such as bone scintigraphy, planar imaging is a common and cost-effective application. Computer processing of the planar scintigrams can increase the accuracy with which the image approximates the activity distribution, selectively enhance normal or abnormal structures of interest, and optimize the use of the display system presenting the image (MacIntyre et al. 1994; Zaidi 2006).
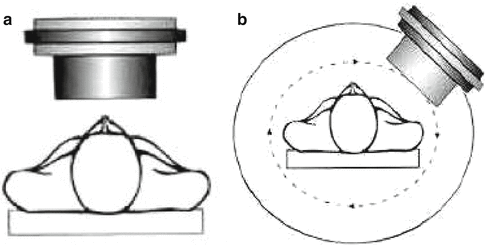

Fig. 1.2
Orientation and patient placement for planar and SPECT imaging. (a) 2D planar scan. (b) 3D SPECT, single-photon emission computed tomography
1.3.2.2 Single-Photon Emission Computed Tomography (SPECT)
SPECT is a 3D tomographic technique that uses the imaging data from many angles which are then reconstructed in different planes. SPECT uses a combination of a rotating gamma camera with a powerful computerized calculation system which allows the acquisition of cross-sectional images (Fig. 1.2b). This technique evolved to three-dimensional imaging acquisition which can be performed within a realistic time frame. SPECT has the capability for mapping physiological function and metabolic activity and thereby providing more specific information concerning organ function for the evaluation of function or physiology. The development of SPECT led to improved imaging of the heart, lung, liver, kidney, bone, and inflamed or infected tissues. SPECT also allows the identification of metastases and determination of the extent of a cancer. SPECT provides the activity distribution in different sections of the object at different depths and in turn to accurately determine the location of the lesion as well as metastasis.
SPECT is a technique whereby cross-sectional images of tissue function can be produced by reconstructing data in slices of the total organ thereby greatly minimizing the removal of the effect of overlying and underlying radioactivity as depicted in Fig. 1.3.
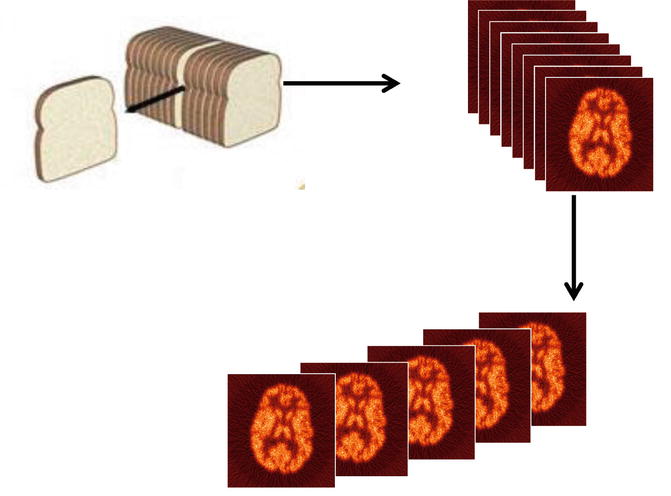

Fig. 1.3
The principal of single-photon emission computed tomography (SPECT)
The functional information obtained by SPECT is complementary to planar images, obtained by projections of the organ under investigation (Eberl et al. 2006; Jaszczak 2006; Horger and Bares 2006; Schillaci 2006; Schillaci et al. 2007; Krausz and Israel 2006). Schematic representation of single-photon emission computed tomography (SPECT) in a clinical setup is depicted in Fig. 1.4. The advantages of SPECT over planar scintigraphy include better spatial localization, improved detection of abnormal function, and, importantly, greatly improved quantification. In general SPECT images have poorer spatial resolution than the 2D images from which they are reconstructed. Of course capital costs and costs for maintenance of SPECT instrumentation are much higher than for planar imaging instruments.
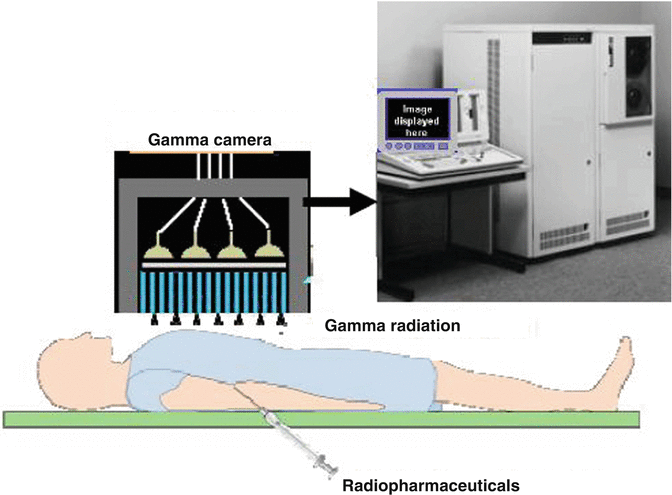

Fig. 1.4
Single-photon emission computed tomography (SPECT)
Radioisotopes used for SPECT are limited to those that emit gamma rays with an energy range that is suitable for the gamma camera such as thallium-201 (201Tl), technetium-99m (99mTc), and iodine-123 (123I). The spatial resolution of SPECT systems is in the range of 10–14 mm.
1.3.2.3 Positron Emission Tomography (PET)
Positron emission tomography (PET) is a powerful diagnostic imaging modality which has become a dominant imaging method in the field of nuclear medicine (Coleman 1999; Gambhir 2002; Delbeke and Martin 2001; Kubota 2001; Mankoff and Bellon 2001; Mammatas et al. 2015; Mercer 2007; Wood et al. 2007; Riemann et al. 2008; Solomon et al. 2003). This technology requires radionuclides that decay with the emission of positrons (β+). The concept of simultaneous detection (coincidence detection) is based on β+ + β−-annihilation by positron interaction with an electron from the surrounding environment after traveling a short distance (3–5 mm), resulting in the emission of two 511 keV gamma rays traveling in opposite directions. In PET, image acquisition is then based on the detection of the two gamma rays in a coincidence mode. A valid annihilation event requires a coincidence within 12 ns between the two detectors placed on opposite sides of the scanner. In a PET instrument, the patient is positioned within a ring of scintillation detectors. If two events are detected simultaneously in opposing detectors, it is assumed that an annihilation occurred somewhere on an imaginary line connecting these two detectors. By acquiring a large number of such events, e.g., 106, tomographic reconstruction methods can be used to reconstruct two-dimensional images of the tracer distribution. Schematic representation of single detector ring of positron emission tomographic (PET) scanner is depicted in Fig. 1.5.
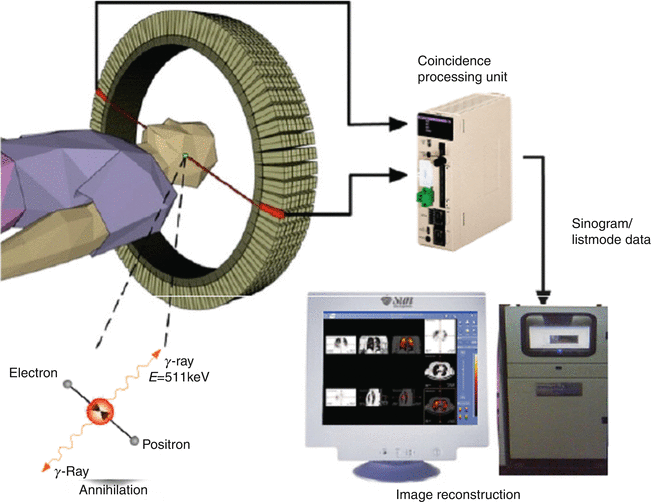





Fig. 1.5
Typical positron emission tomography (PET) system and patient orientation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree