Molecular Imaging
Amir Kashefi
David K. Shelton
Introduction
Molecular imaging has recently been defined as “the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and other living systems.”
In the era of molecular medicine, with genomic maps of humans, small animals, and many pathogens completed, our approach to patient care is being transformed to define underlying molecular and genomic aberrations rather than diseases by clinical signs and symptoms alone. In this era, and specifically in the postgenomic era, in which the functionality of genetic information is the principle of investigations, molecular imaging plays a critical role by identifying, following, and quantifying biologic processes at the molecular level in physiologically intact organisms.
At the preclinical level, molecular imaging is the key for transition from reductionist to a more integrative and holistic approach. This means that traditional in vitro investigations, in which cells are extracted and studied in artificial environments, can now be examined in the context of whole biologic systems in living organisms, by applying molecular imaging techniques. In fact, through molecular imaging, one can observe and follow up dynamic molecular processes in real time by sequential imaging without sacrificing animals serially at fixed time points. This was done to get the tissue for in vitro analysis, which was necessary in traditional in vivo studies.
In clinical settings, molecular imaging has the ability to image specific molecular alterations of underlying diseases, and also to monitor treatment effects at subcellular levels, rather than nonspecific, late morphologic manifestations of these molecular derangements. This ability is a paradigm shift in clinical practice, which allows for earlier and more accurate diagnosis of diseases. One can predict the therapeutic response much earlier, and thus bringing us to an era of personalized medicine.
Molecular imaging exploits diverse methods and concepts, including cellular and molecular biology, multiple imaging modalities, chemistry, physics, nanotechnology, pharmacology, and bioinformatics, to develop innovative diagnostic and therapeutic probes.
Molecular Imaging Modalities
Imaging modalities used in molecular imaging are as follows: PET, SPECT, MRI, US, and optical imaging. Each technology has unique strengths and limitations; Table 61.1 summarizes the attributes of these modalities. Multimodal platforms such as PET-CT, SPECT-CT, PET-MRI, fluorescence-mediated tomography (FMT-CT), and FMT-MRI are emerging to dramatically improve both sensitivity and specificity for each of these modalities.
Coupling these imaging modalities with highly specific probes to serve as sources of imaging signal is the basis of molecular imaging.
Nuclear Imaging
Nuclear medicine developed originally from a confluence of the interests of internal medicine, pathology, and radiology physicians in investigating the physiology of disease. Indeed, nuclear medicine specialists have been practicing molecular imaging since the 1940s when I-131 was identified as the first radiopharmaceutical with clear medical potential and was used for thyroid imaging and therapy. Given its exquisite sensitivity and unlimited depth of penetration, nuclear imaging has always been at the forefront of molecular imaging. PET and SPECT, the most prevalent molecular imaging modalities,
have the ability to locate molecular events in three-dimensional space. PET is generally superior to SPECT because it is more sensitive (about two to three orders of magnitude), has better resolution, and offers better tracer quantification. On the other hand, SPECT is less expensive and utilizes longer-lived radiotracers, thus enabling facile tracer delivery to imaging facilities. PET, on the other hand, has requirements for a cyclotron and a devoted radiochemistry laboratory for many common PET isotopes. SPECT can also have the advantage to be able to distinguish multiple emission energies simultaneously. These attributes have rendered SPECT as a viable molecular imaging choice.
have the ability to locate molecular events in three-dimensional space. PET is generally superior to SPECT because it is more sensitive (about two to three orders of magnitude), has better resolution, and offers better tracer quantification. On the other hand, SPECT is less expensive and utilizes longer-lived radiotracers, thus enabling facile tracer delivery to imaging facilities. PET, on the other hand, has requirements for a cyclotron and a devoted radiochemistry laboratory for many common PET isotopes. SPECT can also have the advantage to be able to distinguish multiple emission energies simultaneously. These attributes have rendered SPECT as a viable molecular imaging choice.
Table 61.1 Overview of Molecular Imaging Modalities | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Radiochemists can potentially label any single biomolecule. Hundreds of PET and SPECT radiotracers have been developed (Table 61.2) and clinically proven over the past two decades, but what stands between these agents and rapid clinical diffusion is political recognition of the very low toxicological risk represented by the use of tracer imaging and of the cost-effectiveness of radiotracer use.
Radioisotopes suitable for molecular imaging may be divided into positron emitters and gamma emitters. Gamma emitters are the isotopes that emit single-photon gamma rays and are employed in standard gamma cameras and SPECT cameras. Positron emitters are employed in PET imaging and always emit two 511 keV photons, which are nearly 180 degrees opposed. Gamma-emitting isotopes are advantageous because they are available with a variety of half-lives, suitable to any purpose. There are several generator systems for convenient production of the desired isotope at the point of consumption. The most commonly utilized generator is the molybdenum-99 (Mo-99)/Tc-99 m generator system.
PET radiopharmaceuticals are very similar chemically to an unlabeled substrate molecule. Gamma emitters are disadvantageous in that they are not easily incorporated into molecules without disrupting their biological function. The “atoms of life” generally do not have gamma-emitting isotopes, although gamma isotopes are available for some less common biometallics, such as selenium-75. Incorporation of gamma emitters into biomolecules generally involves the addition of significant bulk into the molecule. It is difficult to produce a gamma-labeled molecule which has biological behavior identical to that of the original molecule.
Some positron-emitting isotopes have the disadvantage of having a short half-life, thus making the synthesis and use of a positron emitter-labeled substance, a race against time.
The main limitation of PET and SPECT is their low spatial and temporal resolution; however, this has been addressed in the development of hybrid systems with PET and SPECT being coupled with CT. Similarly, combined PET and MRI hybrid systems are currently being developed. Combined PET-MRI may prove better than PET-CT in the detection and evaluation of liver, bone marrow, and CNS lesions. In comparison to CT, MRI also has the advantages of no ionizing radiation and may be acquired simultaneously with the PET data.
MR Imaging
MRI is a powerful imaging modality that provides high-resolution anatomical information, but beyond that, MRI is also very useful for hemodynamic (blood flow and tissue perfusion), metabolic (MR spectroscopy and MRS), functional (fMRI), cellular connectivity (diffusion tensor imaging and DTI), and molecular imaging (using MR-contrast agents).
MRI provides excellent anatomical and spatial resolution, but because of its lower sensitivity compared to PET, SPECT, and optical imaging, MRI’s ability to detect molecular events is somewhat limited, compared to these modalities. Thus, contrast agents are being used to increase MR sensitivity. Contrast agents increase MR signal intensity, by increasing longitudinal and transverse relaxation rates (R1 and R2). Different contrast agents have different effects on R1 and R2. There are two classes of MR-contrast agents: paramagnetic complexes and superparamagnetic iron oxide (SPIO) particles. Although paramagnetic agents equally increase R1 and R2, they are best seen on T1-weighted images since the percentage change in R1 in the tissue is much greater than that in R2. SPIO agents are called T2 agents, since they increase R2 much more than R1, and are best seen on T2-weighted images.
Gadolinium agents currently available in the market are nearly useless for molecular imaging, because they need tissue concentration of 10-7 mol/g to obtain sufficient contrast. This tissue concentration of gadolinium is much higher than that needed for molecular biomarkers. Nanotechnology can help to
overcome this obstacle by incorporating a high payload of a contrast in a single nanoparticle. This increases the effective relaxivity per particle and consequently increases the MR sensitivity.
overcome this obstacle by incorporating a high payload of a contrast in a single nanoparticle. This increases the effective relaxivity per particle and consequently increases the MR sensitivity.
Table 61.2 Examples of Pet and Spect Molecular Imaging Probes and Their Applications | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
However, the challenge with MRI contrasts is also their low specificity. Therefore, these agents need to be targeted to distinct molecules. SPIO agents are prepared in different sizes and of those, ultra-small iron oxide particles (USPIOs, <40 nm) are widely used in molecular imaging. A targeting molecule can be covalently linked to USPIO. Utilizing other approaches such as smart probe technology, and gene reporter imaging, can also improve MRI-contrast agents’ specificity. This will be discussed in more detail later in this chapter.
Magnetic resonance spectroscopy (MRS) estimates the concentration of certain metabolites such as creatine, choline, NAA, lactate, myoinositol, glutamate/glutamine, and lipids, based on chemical shift effect. This means MRS can distinguish different resonance frequencies of nuclei located in different molecular environments (different molecules or different locations within a molecule). MRS studies can be carried out for a single voxel or for a matrix of voxels. 1H MRS, 31P MRS, and 13C MRS are three of the common active nuclei used in MRS studies.
Ultrasound Imaging
US molecular imaging is based on the detection of differential reflections of sound waves from body fluid (mainly consisting of water) and microbubbles of different gases. Microbubbles are US-contrast agents, in the size of 1 to 5 μm, and are composed of a gas (air, sulfur hexafloride, or perfluorocarbons) which is encapsulated by a shell (phospholipid micelles, bilayered membranes, as well as albumin, biocompatible polymers, and other materials). Air dissolves quickly in water or blood and quickly loses its contrast effect. Sulfur hexafloride and perfluorocarbons hardly dissolve in water, behave chemically inert toward biologic systems, and while passing through the capillaries of the lungs, these free gases are exhaled.
Due to the size and mode of delivery, microbubbles are restricted to the intravascular space. Therefore, US molecular imaging is limited to vascular processes such as angiogenesis, inflammation, atherosclerotic plaques, and thrombosis.
Microbubbles can be targeted to specific intravascular epitopes, by modifying the shell properties (phosphatidylserine, phospholipid, or albumin-containing shells) in order to achieve affinity to specific receptors. Affinity is also achieved by attaching specific probes (antibodies, glycoproteins, carbohydrates, or peptides) to the shell.
Imaging with US, however, causes mechanical interaction with the tissue, which may lead to cell destruction and capillary leakage. Microbubbles introduce blood–gas interfaces in the interrogated tissue and can facilitate destructive mechanisms even at FDA-approved diagnostic US energy levels. This raises safety concerns for diagnostic studies, but on the other hand, may be very useful for therapeutic applications. Several studies have shown in vitro and in vivo clot destructive capabilities of microbubbles in thromboembolic disease. US-tissue mechanical interactions can also open cell membranes (sonoporation), which may help targeted drug or gene delivery via loaded microbubbles.
Although US is less sensitive than nuclear imaging and of lower spatial resolution than MR imaging, it offers the advantages of portability, low cost, absence of ionizing radiation, and the potential for therapeutic applications.
Optical Imaging
Optical imaging is based on detecting light photons emitted from imaging probes and travelling through tissue. These photons get scattered and absorbed by tissue components through their pathway to the camera, resulting in significant signal attenuation. Therefore, optical imaging is mainly limited by the depth of photon penetration (<2 cm) through different tissues.
Clinical applications of optical imaging are basically restricted to dermatologic, intraoperative, endoscopic, and intravascular imaging. However, this technique has gained tremendous attention in small animal imaging. High sensitivity, high spatial resolution at limited depth, as well as low cost, simplicity, and favorable safety profile are the advantages that have made optical imaging an ideal method for small animal molecular imaging. Optical imaging can also detect barely emitted visible light from radiotracers and can be useful in translational in vivo research for developing new PET and SPECT tracers. Fluorescence and bioluminescence imaging are the two main optical imaging techniques. Preclinical research is done with the optical molecular compound and then that specific compound can subsequently be labeled with a radiotracer for use in larger animals or humans.
Fluorescence imaging utilizes a fluorophore or fluorescent protein which can be either exogenous or endogenous with inherent optical properties first illuminating by excitation of light at one wavelength and then secondarily emitting light at a shifted wavelength. Emitted light in the visible range (<700 nm) highly scatters and is absorbed by hemoglobin and water. It may overlap with the autofluorescence of tissues, also making it more difficult to detect. On the other hand, the NIR spectral window (700 to 1000 nm) has minimal tissue absorption and minimal autofluorescence, therefore offering improved signal penetration. Fluorescent agents can be classified into three categories: organic (cyanine dyes, porphyrins, chlorines, and phthalocyanines), inorganic (quantum dot particles, carbon dots, and gold nanoparticles), and hybrid (boron-dipyrromethene [BODIPY] and lanthanide chelates).
Bioluminescence imaging depends on the naturally occurring enzyme, luciferase, that converts chemical energy to light, by oxidizing luciferin to an electronically excited molecule which then emits detectable light. Numerous luciferase–luciferin pairs are now being used for in vivo imaging. Bioluminescence imaging is more sensitive compared to fluorescence techniques since there is no inherent background noise.
The major drawback for clinical optical imaging is poor deep-tissue penetration. However, recently developed advanced techniques such as fluorescence-mediated tomography (FMT) and photo-acoustic tomography (PAT), as well as new nanomedicine concepts will bring more attention to optical imaging in the near future.
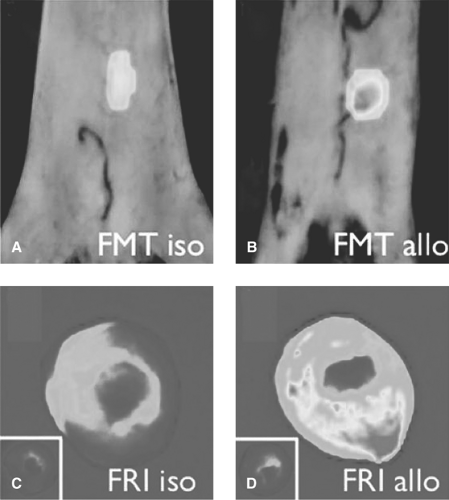
FMT reconstructs tomographic images of fluorescence acquisition signals by using sophisticated algorithms (Fig. 61.1). FMT is a quantitative system that can improve spatial resolution and achieve depth penetrations as great as 10 cm. Hybrid systems such as FMT-CT and FMT-MRI are beneficial for improved photon reconstruction and image visualization.
Photo-acoustic imaging is a new imaging technology that brings together the advantages of US and optical imaging. PAT exploits a short-pulsed laser source to irradiate tissue and temporarily raise its temperature (by millikelvins), which subsequently causes thermoelastic expansion of the tissue. This tissue thermoelastic effect prompts acoustic wave propagation that can be detected by wide-band ultrasonic transducers.
The magnitude of the ultrasonic emission is proportional to the absorbed light by different tissues. Hemoglobin (blood) and melanin (melanoma) are two of the endogenous molecules that can provide sufficient contrast from surrounding tissues and can be detected by PAT. Therefore, exogenously administered contrast-enhancing agents that have affinity for specific molecules are needed for PAT molecular imaging.
Gold nanoparticles and gold nanobeacons, which incorporate multiple tiny gold nanoparticles within a bigger vascularly constrained nanoparticle, are becoming prime contrast agents for PAT molecular imaging.
Cerenkov Luminescence Imaging (CLI). The speed of light decreases by about 25% when it travels through water. A charged particle that can move faster than the light in water can create an electromagnetic “shock wave” (much like an airplane that travels faster than the speed of sound). This electromagnetic shock wave appears as a visible blue light known as “Cerenkov radiation.”
Cerenkov imaging is a type of optical imaging, which exploits radioactivity as the source of light. This technique
is specifically promising in imaging radionuclides that do not emit either positrons or gamma rays—a current limitation for nuclear imaging modalities.
is specifically promising in imaging radionuclides that do not emit either positrons or gamma rays—a current limitation for nuclear imaging modalities.
Molecular Imaging Strategies
Molecular imaging can be categorized based upon two different practicing strategies of direct and indirect molecular imaging.
Direct Molecular Imaging
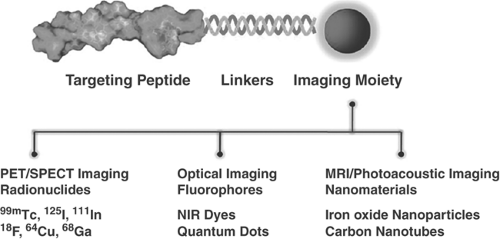
Direct molecular imaging is based on imaging a molecular target via a highly specific probe that has great affinity for the intended target. Targets can be anywhere from within the cell membrane to the cell nucleus. Probes are designed molecules, composed of any of the imaging system contrast agents and a binding moiety with high affinity for the target (Fig. 61.2).
Binding affinity, specificity, molecular weight, clearance rates, excretion routes, and toxicity are the important features that biochemists consider when designing a probe. A general rule is that the higher the target to background ratio at the time of imaging, the higher the sensitivity for detection of the pathologic process. Two approaches in use are specific monoclonal antibodies and affibody molecules.
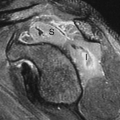
Specific monoclonal antibodies are labeled with radiotracers or contrast agents. However, due to their long biodistribution time and slow blood clearance, there is high background signal, and so their applications are limited. Thus, smaller antibody fragments such as antigen-binding fragments (Fab), and single-chain variable fragments (scFv) are superior over intact immunoglobulin. Smaller fragments will be advantageous, and often required, for isotopic labels having relatively short half-lives. Smaller fragments are also less immunogenic, and thus less likely to induce an undesirable immune response. Larger fragments or intact antibodies may be preferable where a long reaction time is required, such as when binding is weak or slow. Capromab pendetide (ProstaScint®) is an example of radiolabeled (111In) monoclonal antibody to prostate-specific membrane antigen used in prostate cancer imaging (Fig. 61.3). Larger fragments may also be better for therapeutic applications where extended residence time in the target tissue is required to maximize radiation dose to the tumor.
The use of smaller fragments for imaging results in significantly increased renal activity. This can be addressed by the administration of unlabeled leucine, which saturates the renal binding sites for the fragments and reduces their residence time in the kidneys. Mixtures of amino acids, commercially available for total parenteral nutrition, appear to work just as well. Another approach to limiting renal activity is to use smaller fragments consisting only of the active binding site, or even of fragments of this active site.
Affibody Technology. Affibody molecules are small scaffold proteins that are completely unrelated to the antibodies. The origin of affibody molecules is from the domain B of staphylococcus aureus protein A. Affibody molecules are able to bind different targets, have rigid structures with rapid folding properties that tolerate modifications and conjugations, and are very small (∼7 kDa) molecules. In addition, the absence of cysteines in affibody molecules allows direct incorporation of radionuclide chelators. HER2-binding affibodies are the most common affibody probes. Several HER2-binding affibody probes have been designed for PET, SPECT, and optical imaging. These include 18F-FBEM-ZHER2:342, affiprobes (consisting of a HER2- or EGFR-specific affibody molecule and a fluorescent moiety, mCherry [red], or EGFP [green]), 99mTc-MAG3-ZHER2:342,
99mTc-CGG-ZHER2:342, and 68Ga-ABY-002 (Fig. 61.4) which are all currently at the preclinical level.
99mTc-CGG-ZHER2:342, and 68Ga-ABY-002 (Fig. 61.4) which are all currently at the preclinical level.
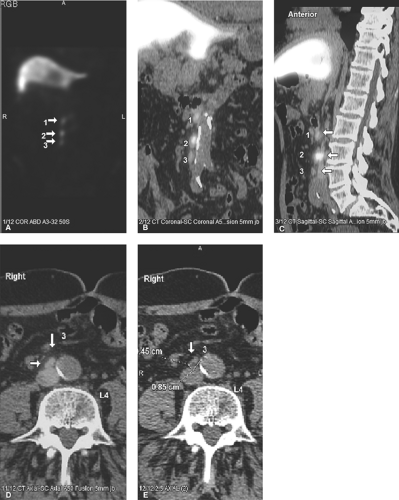
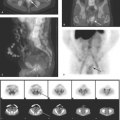
Natural peptide receptor ligands (cytokines and chemokines) are endogenous molecules with defined jobs like cross-talk between immunocompetent cells, upregulation of adhesion molecules, wound healing, and control of the neoplastic process. These characteristics have made them interesting imaging probes. It is important to administer a small amount (a few micrograms) of cytokines for imaging purposes, since they may induce biological effects. Several peptide receptor ligands, such as VEGF (angiogenesis), IL2 (inflammation), EGF (breast and squamous cell lung cancers), Annexin V (apoptosis), and octreotide (neuroendocrine tumors) have been studied for molecular imaging. Of these, In-111-DTPA-D-Phe1-octreotide (In-111-OCT) with high affinity for the sst2 somatostatin receptor has been widely used clinically. In-111-OCT has greater than 90% sensitivity for the detection of carcinoid tumors. It can also be used to detect gastrinomas, insulinomas, glucagonomas, and other (unclassified) APUDomas (Fig. 61.5). It detects primary small cell lung carcinoma with 90% sensitivity and nonliver metastases from small cell lung carcinoma with 70% sensitivity. It also has fair sensitivity for medullary thyroid carcinoma (65%). Recently, 68 Ga-DOTA-somatostatin receptor analogues (PET tracers) such as 68Ga-DOTA-NOC and 68Ga-DOTA-TATE have been shown to have higher sensitivity and specificity for neuroendocrine tumor imaging (Fig. 61.6). Some of these somatostatin analogs have also been labeled with beta emitting radionuclides such as Yttrium-90 and lutetium-177, as a new tool for metastatic neuroendocrine tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree