Ovarian Carcinoma
Todd M. Blodgett, MD
Alex Ryan, MD
Hesham Amr, MD
Key Facts
Terminology
Ovarian cancer, carcinoma of the ovary
Imaging Findings
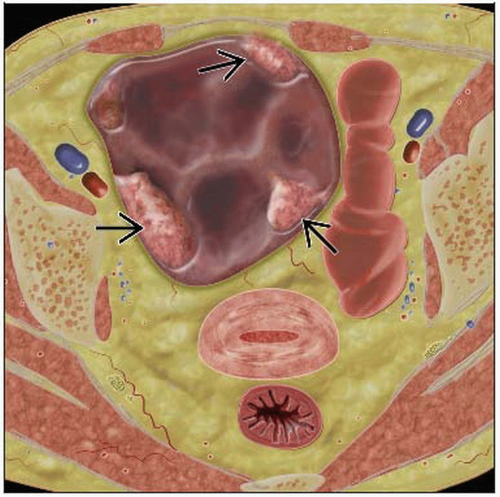
Solid or complex cystic mass arising from ovary ± ascites
Metastases most common to peritoneum, omentum
Primary lesion usually complex cystic mass with mural nodularity
In general, malignancy suggested by thickness and irregularity of cavity walls, septae, enhancing nodules
Sensitivity 92% for peritoneal metastases
Pure mucinous adenocarcinoma more likely to be falsely negative by standard SUV criteria due to low cellularity and better differentiation properties
PET limited in detecting lymph node micrometastases
In one study, PET/CT failed to identify microscopic disease in 59% of pathologically positive lymph nodes
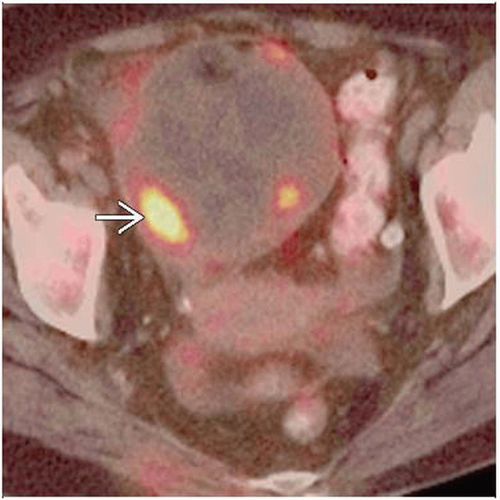
PET/CT: Sensitivity > 95%, specificity 80-93%, and positive predictive value of 83-94% for detection of recurrence
Top Differential Diagnoses
Pelvic Inflammatory Disease
Tubo-Ovarian Abscess
Complex Functional Cysts
Benign Ovarian Tumors
Borderline Ovarian Tumors
Diagnostic Checklist
PET/CT valuable in patient with rising CA-125, negative anatomic imaging
TERMINOLOGY
Abbreviations and Synonyms
Ovarian carcinoma, carcinoma of the ovary, ovarian cancer
Definitions
Primary malignancy of ovary
Epithelial (90%)
Arises from germinal epithelium on outside of ovary
Stromal (6%)
Arises from connective tissue
Low rate of metastasis
Germ cell (3%)
Teens/young women
Highly curable
Staging of ovarian cancer
Stage I: Confined to one or both ovaries
Stage II: Spread to uterus/fallopian tube, within pelvis
Stage III: Lymph nodes, abdominal cavity
Stage IV: Outside abdomen, intrahepatic metastases
IMAGING FINDINGS
General Features
Best diagnostic clue: Solid or complex cystic mass arising from ovary ± ascites
Location
Primary generally found in ovary
Metastases most common to peritoneum, omentum
Rare intrahepatic metastases
Three main routes of lymphatic spread
Accompanying ovarian blood vessels cranially to para-aortic and paracaval nodes
Following subovarian plexus in broad ligament to obturator and pelvic nodes
Following round ligament to external iliac and inguinal nodes
Size
Variable
However, most ovarian carcinoma detected late, so tumors tend to be large
Imaging Recommendations
Best imaging tool
Most common: Transvaginal ultrasound followed by MR &/or CT for evaluation of metastasis
Risk of malignancy index (RMI) calculated using transvaginal ultrasound results, CA-125 blood level, menopausal status
Consider PET/CT for most complete staging
Especially use PET/CT for suspected recurrence, particularly with mild rise in CA-125
Protocol advice: Consider using contrast-enhanced CT as part of the PET/CT scan
CT Findings
Primary lesion usually complex cystic mass with mural nodularity
In general, malignancy suggested by thickness and irregularity of cavity walls, septae, enhancing nodules
Sensitivity 92% for peritoneal metastases
GI contrast especially helpful for distinguishing pelvic viscera from intestinal tract
Primary use of CT scanning is to evaluate metastatic disease, not ovarian mass
For evaluation of the ovarian mass, ultrasonography and MR are more valuable
CT scanning is helpful in diagnosing cystic teratomas, 93% of which contain fat and 56% of which are calcified
If a large (> 10 cm) soft tissue mass is present, malignant transformation should be suspected
Serous cystadenoma has an attenuation similar to that of water
But mucinous cystadenoma has an attenuation closer to that of soft tissue
CT imaging has much greater sensitivity than techniques used previously
Detects 2-3 mm lesions in the lungs and solid viscera
Scans with contrast yield high-quality information about retroperitoneal lymph nodes and ureters
Low contrast between peritoneal tumors and adjacent soft tissues limits overall sensitivity
MR sensitivity in this case 91%
But limited in depiction of small calcified peritoneal implants, which are common in patients with serous carcinoma
NECT
Cystic adnexal mass with septations and soft tissue density papillary projections
CECT
Solid mural nodules demonstrate enhancement
May facilitate detection of peritoneal implants and distant metastases
CT angiogram: Can be used to assess vascular invasion
Nuclear Medicine Findings
Initial diagnosis
FDG PET almost never used to evaluate an adnexal mass or to evaluate for primary ovarian carcinoma
Pure mucinous adenocarcinoma more likely to be falsely negative by standard SUV criteria, due to low cellularity and better differentiation properties
Staging
Not currently covered by Medicare
Three lymph node stations with high rate of false positives: Axillary, inguinal, hilar
PET limited in detecting lymph node micrometastases
In one study, PET/CT failed to identify microscopic disease in 59% of pathologically positive lymph nodes
Also limited for differentiating peritoneal tumors from adjacent soft tissue or bowel activity
Restaging
PET/CT: Sensitivity > 95%, specificity 80-93%, and positive predictive value of 83-94% for detection of recurrence
PET has 6-8 mm limit of resolution
Limited in detection of small disseminated lesions (e.g., peritoneal carcinosis and mesenteric or omental recurrences)
Degenerative change in pelvis, such as sacroiliac arthritis, has been mistaken for recurrence
Active degenerative change in bone can have ↑ FDG activity
For detection of ovarian cancer recurrence mean sensitivity, specificity, and accuracy each 83%
In one study of patients with primarily subcentimeter lesions, sensitivity of FDG PET for recurrence was only 10%
With mean lesion size of 1.1 cm, patient-based sensitivity 81%
Response to therapy
Paucity of studies in the literature; not currently covered or recommended for evaluating response to therapy
DIFFERENTIAL DIAGNOSIS
Pelvic Inflammatory Disease
CT findings nonspecific
Disrupted fat planes
Thickened fascial planes
Tubo-Ovarian Abscess
Commonly depicted as regular mass with debris similar to that seen with endometrioma or hemorrhagic cyst
Complex Functional Cysts
May have intense FDG activity
Benign Ovarian Tumors
Includes cystadenoma, dermoid tumors
Borderline Ovarian Tumors
Pathologically difficult to differentiate between benign and malignant
Low malignant potential
Normal Physiologic FDG Activity
Mostly in younger premenopausal women
Helpful if bilateral physiologic activity present
Look for CT findings of corpus luteal cyst: Rind of enhancement in an otherwise normal-appearing ovary
PATHOLOGY
General Features
General path comments
Ovarian cancer spreads primarily intraperitoneally as well as to lymph nodes
Peritoneal fluid flows upward from pelvis to paracolic gutters and subphrenic regions, carrying tumor cells that implant on abdominal viscera
Common sites of metastatic implantation
Pelvis
Right hemidiaphragm
Perihepatic
Right paracolic gutter
Bowel
Omentum
Distant lymph nodes are involved in approximately 7% of cases of serous ovarian adenocarcinoma
Genetics
10% of patients with ovarian cancer appear to have genetic predisposition
These patients may develop cancer early, between ages 30 and 50
One study suggested patients with BRCA gene have 60% risk of developing ovarian cancer
Etiology
Unknown
Number of reproductive cycles appears to be related to risk
Ovulation suppression may decrease cancer incidence
Epidemiology
Leading cause of death among women with gynecological malignancies
Third most common cancer of female reproductive organs
Microscopic Features
Most common histologies are papillary serous adenocarcinoma and endometrioid type
Serous adenocarcinoma comprises 40% of epithelial ovarian cancers
Psammoma bodies may be present
Ovarian cancer with multiple psammoma bodies may have better prognosis
CLINICAL ISSUES
Presentation
Most common signs/symptoms
Early stage: Nonspecific, pelvic pain
Often attributed to other causes (e.g., menstruation, irritable bowel syndrome)
With metastases: Abdominal/pelvic bloating, pain, pressure, early satiety, nausea/vomiting, frequent urination, feeling similar to pregnancy
Other signs/symptoms
CA-125 has accuracy of 79-95% for recurrence; increase precedes apparent recurrence by 3-6 months
Doubling of CA-125 above normal limit has been shown to have sensitivity of 85.9% and specificity of 91.3% for detection of recurrence
Demographics
Age: Average age at diagnosis 57 years
Ethnicity: More common among Caucasians than African-Americans
Natural History & Prognosis
Most patients asymptomatic until disease is in advanced stage
In 75-80% of patients, cancer has spread beyond ovary at diagnosis
Overall survival approximately 35%
Despite clinical advances and improved surgery, overall survival has not changed because the disease presents at advanced stage
75% of patients are diagnosed in stage III/IV, and in this group the 5 year survival rate is 17%
Up to 85% of women ultimately relapse
Well-known that patients with advanced ovarian cancer have better outcome when neoadjuvant chemotherapy is performed before surgery
Up to 24% of ovarian tumors in premenopausal women are malignant and up to 60% in postmenopausal women
Treatment
Surgery, chemotherapy, radiation therapy; depends on stage of disease, institution where treated
Surgery for earlier stages: Total abdominal hysterectomy, bilateral oophorectomy, omentectomy, biopsy of lymph nodes/tissues
Surgery for later stages: Early stage surgery plus tumor debulking
Chemotherapy: Paclitaxel &/or platinum-based drugs
Radiation therapy: Stage II
Exploratory laparotomy done for high suspicion of malignancy
Only 1 ovarian cancer detected for every 8-9 benign cyst operations
Benefit of optimal primary cytoreductive debulking surgery is well established
Following debulking surgery, stage IV patients have same median survival as stage III patients
75% of women have complete clinical response, but the majority of these will experience recurrence
DIAGNOSTIC CHECKLIST
Consider
Benign increased FDG uptake in ovaries can mimic ovarian primary malignancy
Look for other signs of ovarian malignancy (ascites, peritoneal implants, lymphadenopathy)
PET/CT valuable in patient with rising CA-125, negative anatomic imaging
Some claim PET is as valuable for detection of recurrence as second-look surgery and may be substituted as noninvasive option
PET/CT valuable to distinguish post-surgical change from recurrence
Image Interpretation Pearls
Serous adenocarcinoma may contain microcalcifications that can be confused with old granulomatous disease
Incidence of groin metastases less than 3%; isolated inguinal nodal metastasis without other nodal involvement very rare
SELECTED REFERENCES
1. Hillner BE et al: Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol. 26(13):2155-61, 2008
2. Reinhardt MJ: Gynecologic tumors. Recent Results Cancer Res. 170:141-50, 2008
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree