Figure 1-14. Patterns of pain commonly referred to shoulder US. Pink= typical location for the perception of pain related to rotator cuff disease. Light gray= areas of pain unrelated to rotator cuff disorders.
7.1. Tendinopathy
At US, tendinopathy is depicted as a thickened and/or hypoechoic tendon. Echogenicity is assessed subjectively and the tendon considered hypoechoic when iso- or hypoechoic when compared to the deltoid muscle (figure 1-15). In order to avoid anisotropy, tendon echogenicity should be evaluated only when insonating beam is perpendicular to the tendon fibers. Evaluation of tendon thickness is more objective and the normal supraspinatus tendon should not exceed 6 mm when measured orthogonal to the bone cortex (figure 1-16). In some individuals, the affected tendon also shows effacement of the fibrillar pattern (figure 1-17). Contralateral comparison is not obligatory but can assist in difficult cases by revealing asymmetric tendon thickness (figure 1-18). Tendon asymmetry, however, is not a requisite for the diagnosis (figure 1-19). It is important to remember that the anterior portion of the supraspinatus tendon is thicker than the wider and thinner posterior portion because load and stress are significantly higher anteriorly, and contralateral comparison should be made at similar levels (figure 1-20). The key point to avoid inadequate comparison is to recognize that similar tendon portions have similar bone contour, and contralateral comparison is adequate only when identical bone contour is obtained. Although most commonly seen in the supraspinatus, tendinopathy may also occur in the subscapularis (figure 1-21), infraspinatus (figure 1-22), and teres minor tendons. The diagnosis of supraspinatus or subscapularis tendinopathy should prompt a careful dynamic scrutiny of subacromial (video 1-3) and subcoracoid impingement (video 1-4), respectively.
Doppler US may be useful to depict neovascularity, which correlates with clinical findings161 and improves confidence in the diagnosis (figure 1-23). A common misconception is that increased blood flow reflects an inflammatory condition, when, in fact, it represents an inflammatory response that can be found in both inflammatory and degenerative conditions.162-166 In chronic degenerative disorders, such as tendinopathy, increased blood flow reflects active reparative process (video 1-5). The two major drawbacks of Doppler US are that it does not modify treatment and negatively impact three advantageous features of conventional US since it increases costs, lowers availability, and increases time of execution. The cost-benefit of adding Doppler US to routine clinical practice is yet to be defined.
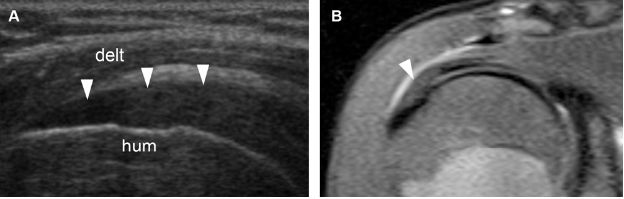
Figure 1-15. Supraspinatus tendinopathy. [A] Long-axis 12-5 MHz US image shows hypoechoic supraspinatus tendon (arrowheads). [B] Corresponding coronal oblique STIR MRI confirms tendinopathy of the supraspinatus (arrowhead). Hum= humerus. Delt= deltoid.
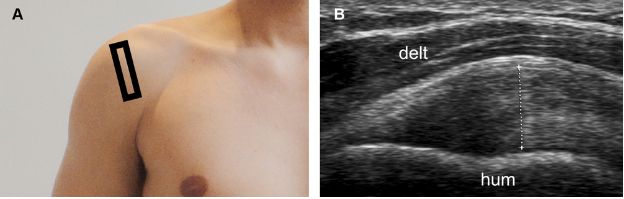
Figure 1-16. Supraspinatus tendinopathy. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows thickening of the supraspinatus tendon, estimated at 8 mm (cursors). The measurement should be obtained at the point of maximal tendon thickness and must neither include the articular cartilage nor the peribursal echogenic fat. Hum= humerus. Delt= deltoid.
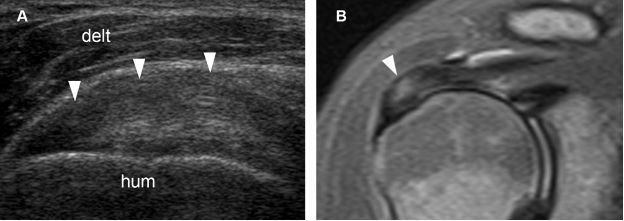
Figure 1-17. Supraspinatus tendinopathy. [A] Long-axis 12-5 MHz US image shows hypoechoic tendon thickening and loss of fibrillar pattern (arrowheads). [B] Corresponding coronal oblique STIR MRI confirms tendinopathy of the supraspinatus (arrowhead). Hum= humerus. Delt= deltoid.
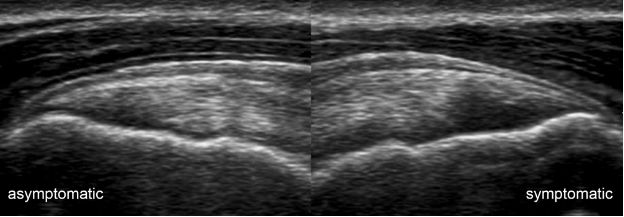
Figure 1-18. Supraspinatus tendinopathy. Long-axis 12-5 MHz comparative image shows asymmetric supraspinatus tendon thickness due to tendinopathy in the symptomatic side.
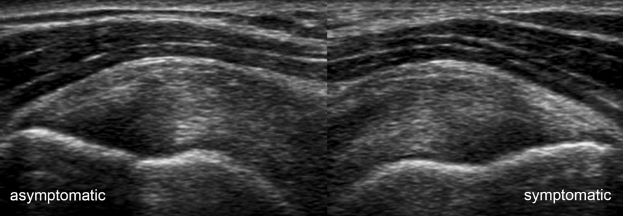
Figure 1-19. Supraspinatus tendinopathy. Long-axis 12-5 MHz comparative image shows symmetric thickening of the supraspinatus due to bilateral tendinopathy despite unilateral symptoms.
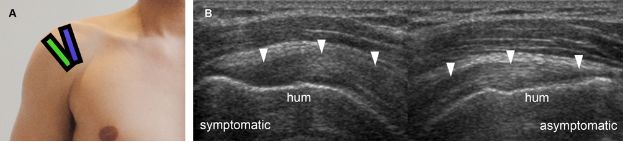
Figure 1-20. Supraspinatus portions. [A] Positioning of the probe to long-axis evaluation of the anterior (blue) and posterior (green) portions of the supraspinatus tendon. [B] Corresponding long-axis 12-5 MHz comparative US shows asymmetric supraspinatus thickness (arrowheads) because image of symptomatic side corresponds to anterior portion while image of asymptomatic side corresponds to posterior portion of the tendon. Note that different portions also have different bone contour. Hum= humerus.
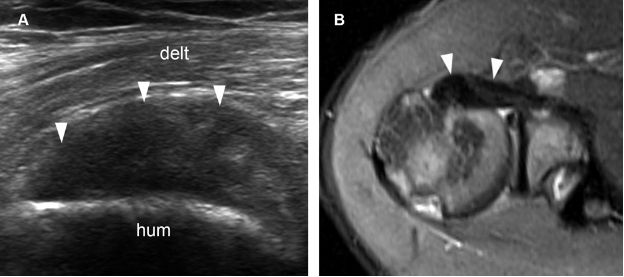
Figure 1-21. Subscapularis tendinopathy. [A] Long-axis 12-5 MHz US image shows hypoechoic thickening of the subscapularis tendon and loss of fibrillar pattern (arrowheads). [B] Corresponding axial STIR MRI confirms subscapularis tendinopathy (arrowheads). Hum= humerus. Delt= deltoid.
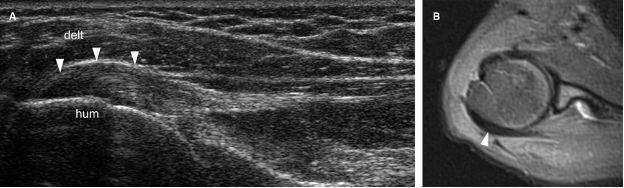
Figure 1-22. Infraspinatus tendinopathy. [A] Long-axis 12-5 MHz US dual-image shows hypoechoic thickening of the infraspinatus tendon (arrowheads). [B] Corresponding axial STIR MRI confirms infraspinatus tendinopathy (arrowhead). Hum= humerus. Delt= deltoid.
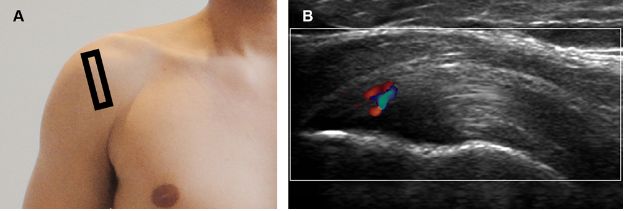
Figure 1-23. Supraspinatus tendinopathy. [A] Positioning of the probe. [B] Corresponding 12-5 MHz Doppler US image shows thickened supraspinatus tendon with increased vascularity.
7.1.1. Grading of Tendinopathy
We generally do not report measurements of tendon thickness. We also do not subjectively report tendinopathy as mild, moderate or severe. To the best of our knowledge, studies correlating sonographic grades of tendinopathy to pain or functional prognosis are lacking. The decision to return patients to the baseline level of physical activity should be based on clinical grounds, not on sonographic findings.
7.1.2. Follow-up of Tendinopathy
The primary objective of the treatment is not to restore normal anatomy but to halt or slow the progression of the disease and offer symptomatic relief. Tendinopathy is not like sinusitis, which resolves after successful treatment. As a predominantly degenerative disorder, tendinopathy is much more like an osteophyte and should be viewed as a permanent lesion, which does not eventually disappear over time (figure 1-24). Follow-up studies are probably best reserved for clinically refractory patients at risk for progression to a tear, because informing a good responder that he still has a lesion may cause symptoms to recur.
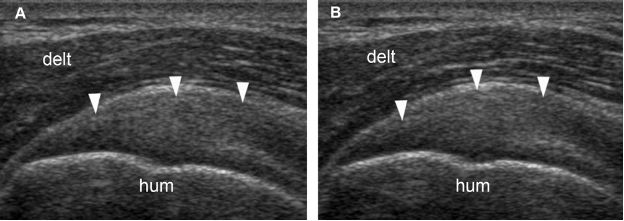
Figure 1-24. Follow-up of supraspinatus tendinopathy. [A] Baseline long-axis 12-5 MHz US image shows thickened supraspinatus tendon with loss of fibrillar pattern (arrowheads). [B] Follow-up long-axis 12-5 MHz US image obtained forty-five days later shows no change in sonographic findings (arrowheads). The patient has become asymptomatic during the first week of treatment. Hum= humerus. Delt= deltoid.
7.2. Calcific Tendinopathy
Rotator cuff calcific tendinopathy is frequent and reported in 8 to 20% of adults on the basis of radiographs.167 Clinically, patients may be asymptomatic or may present with either acute or chronic pain.
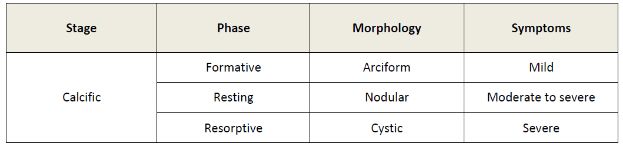
The sonographic appearance of calcific tendinopathy is variable (table 1-4).168 In the formative phase, calcium deposits are depicted as an arciform echogenic structure with marked acoustic shadow (figure 1-25) or fragmented thin echogenic foci with variable shadowing (figure 1-26). In the resting phase, calcium deposits are thicker, more nodular, and typically show no shadowing (figure 1-27). Finally, in the resorptive phase, calcium deposits show bold echogenic wall surrounding a hypo-anechoic area or layering content (figure 1-28). Increased vascularity may be observed at any stage and reflects active reparative process (figure 1-29; videos 1-6 and 1-7). It is believed that the calcific tendinopathy becomes more symptomatic and vascularized when the calcium deposit undergoes resorption.169 Symptoms at this stage include severe pain and restriction of movement, usually for about two weeks.169
Table 1-4. Correlation of sonographic phase, morphology and symptoms during calcific stage of calcific tendinopathy.
Calcific tendinopathy involves supraspinatus, infraspinatus (figure 1-30), subscapularis (figure 1-31), and teres minor (figure 1-32) in decreasing order of frequency. Calcifications located in the subscapularis, infraspinatus (video 1-8), or teres minor tendons may be difficult to demonstrate radiographically because of the overlapping shadow of the humerus (figure 1-33). Calcium deposits may likewise be overlooked on MRI because it is not easily distinguished from the tendon. The reason why some calcium deposits involve the main body of the tendon, while others are insertional (figure 1-34) or even protrude from the tendon and collect beneath the subacromial-subdeltoid bursa is not clear (figure 1-35; video 1-9).
The erosive form is considered a variant presentation of calcific tendinopathy that is associated to poorer outcomes,170,171 and believed to result from active inflammation at the tendon insertion induced by calcium deposits (figure 1-36).172 Imaging findings may resemble those observed in rim-rent tears (see section 7.3), but the calcific material is usually thicker and longer when compared to the stria associated to partial-thickness tears (figure 1-37).
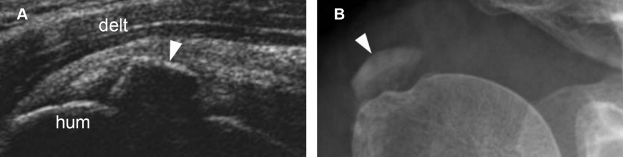
Figure 1-25. Calcific supraspinatus tendinopathy in the formative phase. [A] Long-axis 12-5 MHz US image shows a thin arciform echogenic focus with acoustic shadowing within the supraspinatus tendon (arrowhead). [B] Corresponding radiography confirms sonographic finding (arrowhead). Hum= humerus. Delt= deltoid.
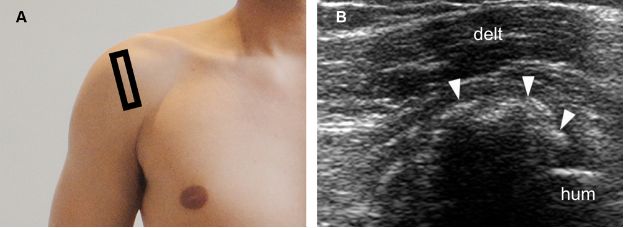
Figure 1-26. Calcific supraspinatus tendinopathy in the formative phase. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows fragmented thin echogenic foci with acoustic shadowing within the supraspinatus tendon (arrowheads). Hum= humerus. Delt= deltoid.
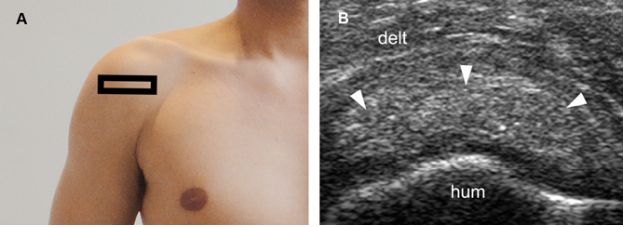
Figure 1-27. Calcific supraspinatus tendinopathy in the resting phase. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a thick nodular calcification with no acoustic shadowing within the supraspinatus tendon (arrowheads). Hum= humerus. Delt= deltoid.
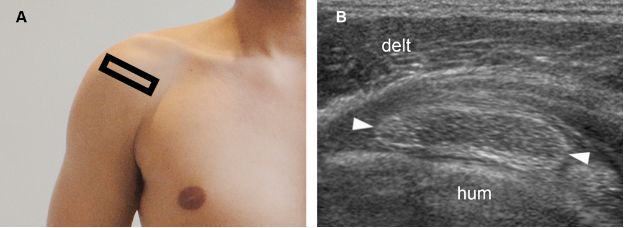
Figure 1-28. Calcific supraspinatus tendinopathy in the resorptive phase. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a hypoechoic area surrounded by bold echogenic wall (arrowheads). Hum= humerus. Delt= deltoid.
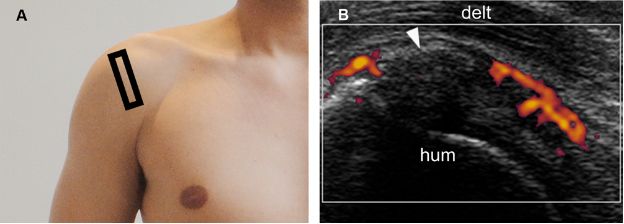
Figure 1-29. Calcific supraspinatus tendinopathy in the formative phase. [A] Positioning of the probe. [B] Corresponding 12-5 MHz power Doppler US image shows increased vascularity adjacent to a thin arciform calcification (arrowhead) within the supraspinatus tendon. Hum= humerus. Delt= deltoid.
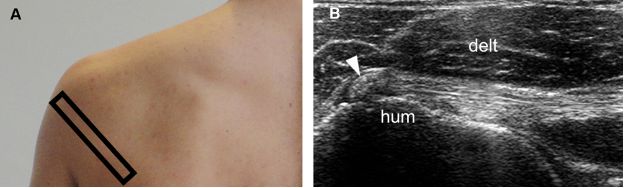
Figure 1-30. Calcific infraspinatus tendinopathy. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a calcific focus within the infraspinatus tendon (arrowhead). Hum= humerus. Delt= deltoid.
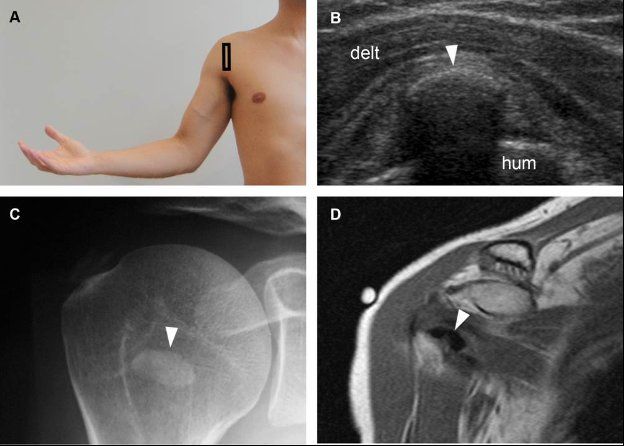
Figure 1-31. Calcific subscapularis tendinopathy. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows an arciform echogenic focus with shadowing within the subscapularis tendon (arrowhead). [C] Corresponding radiography confirms sonographic finding (arrowhead). [D] Corresponding coronal oblique T1-weighted MRI also confirms calcific subscapularis tendinopathy (arrowhead). Hum= humerus. Delt= deltoid.
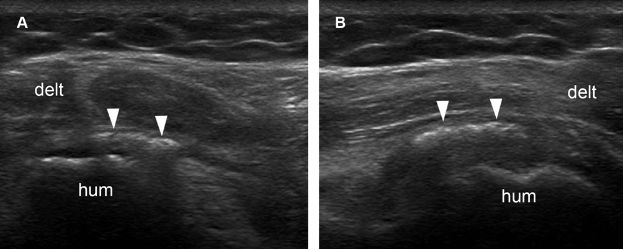
Figure 1-32. Calcific teres minor tendinopathy. [A] Long-axis 12-5 MHz US image shows an echogenic focus with no shadowing within the teres minor tendon (arrowheads). [B] Short-axis 12-5 MHz US image confirms long-axis finding. Hum= humerus. Delt= deltoid.
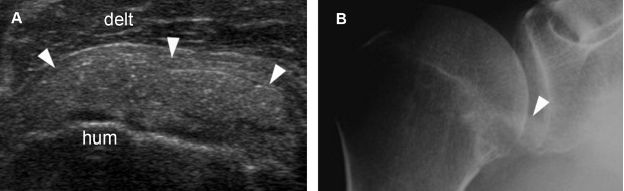
Figure 1-33. Calcific subscapularis tendinopathy. [A] Long-axis 12-5 MHz US image depicts a thick nodular calcification with no shadowing within the subscapularis tendon (arrowheads). [B] Corresponding radiography shows a faint calcification in the region of subscapularis tendon (arrowhead). Hum= humerus. Delt= deltoid.
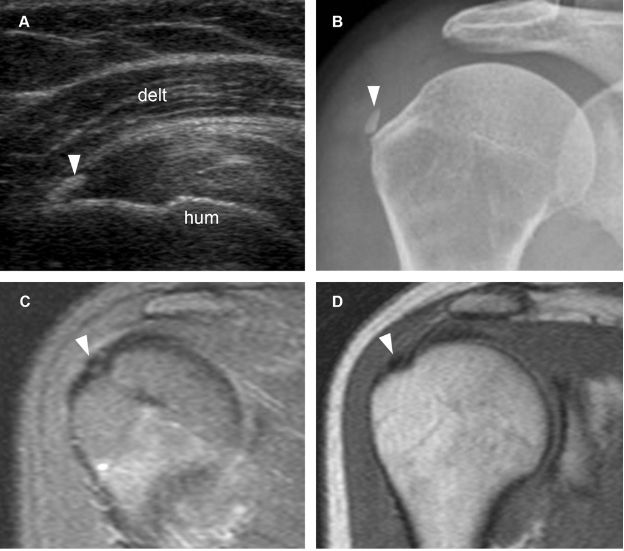
Figure 1-34. Calcific supraspinatus tendinopathy. [A] Long-axis 12-5 MHz US image shows a thin echogenic focus at the distal insertion of the supraspinatus tendon (arrowhead). [B] Corresponding radiography confirms sonographic finding (arrowhead). [C] Corresponding coronal oblique STIR MRI shows the signal void of calcium deposit (arrowhead). [D] Corresponding coronal oblique T1-weighted MRI confirms the signal void (arrowhead). Hum= humerus. Delt= deltoid.
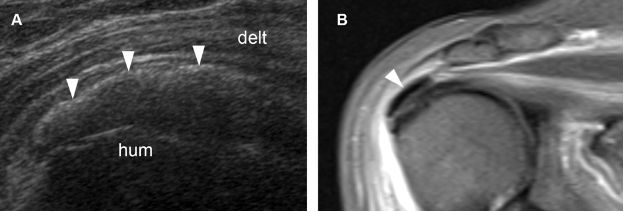
Figure 1-35. Calcific supraspinatus tendinopathy. [A] Long-axis 12-5 MHz US image shows an arciform calcification with faint shadowing protruding from the supraspinatus tendon (arrowheads). [B] Corresponding coronal oblique STIR MRI confirms calcium deposit protruding from the supraspinatus tendon (arrowhead). Hum= humerus. Delt= deltoid.
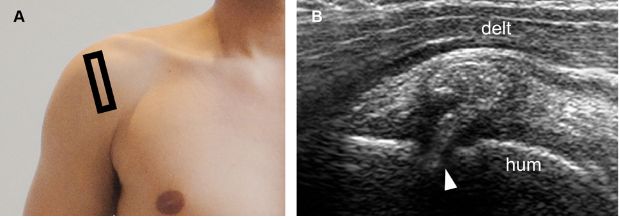
Figure 1-36. Erosive calcific supraspinatus tendinopathy. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows cortical erosion at the point where a thick calcification with no shadowing meets the humeral head (arrowhead). Hum= humerus. Delt= deltoid.
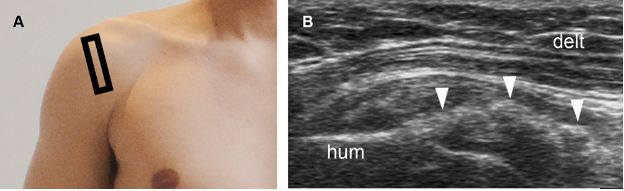
Figure 1-37. Erosive calcific supraspinatus tendinopathy. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a thick calcification with no shadowing in contact with a cortical erosion at the greater tuberosity (arrowhead). Hum= humerus. Delt= deltoid.
7.2.1. Grading of Calcific Tendinopathy
The most intuitive way of grading calcific tendinopathy is to report the size of calcium deposit, and there is some evidence to suggest that the it may correlate with shoulder pain in both preoperative173 and postoperative setting.174 However, this relationship is not free of controversy, especially because anecdotal experience during contralateral comparison suggests that the size of calcium deposit does not correlate with symptoms in many individuals. As a prognostic marker, the preoperative size of calcium deposit is irrelevant and clinical response after surgery correlates only with the absence of residual calcification.173
Hence, we generally do not report the size of calcium deposit because (1) there is no solid evidence indicating that this information is clinically relevant for management, (2) it induces the referring physician to mathematize the condition for follow-up, and (3) sonographic measurements may not correlate with the volume of calcium because of shadowing (figure 1-38). We, however, do our best to report the stage of calcific tendinopathy because it correlates with clinical symptoms and may influence management, but this can be challenging since the abnormality is often multifocal and not necessarily synchronic.175
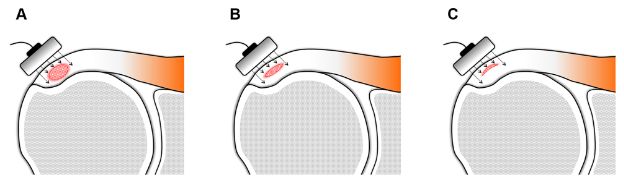
Figure 1-38. Calcific supraspinatus tendinopathy. Schematic drawing of a coronal view of the shoulder shows that sonographic measurements may not correlate with the volume of calcific deposit because of shadowing. [A,B,C] All calcifications have the same mediolateral and anteroposterior diameter, but volumes vary considerably because of different thickness.
7.2.2 Follow-up of Calcific Tendinopathy
Serial evaluation of calcific tendinopathy reveals a predictable pattern of morphologic changes, from precalcific to calcific and postcalcific stages. In the calcific stage, there is also a predictable pattern of changes, from formative to resting and resorptive phases. A prolonged time frame is typically required to obviate these changes, in the range of several months, not just few weeks.
7.3. Partial-Thickness Tears
Partial-thickness tears are defined as a disruption of tendon fibers that does not allow communication between the glenohumeral joint cavity and the subacromial-subdeltoid bursa. Partial tears may be intrasubstance, or extend to either the bursal or articular surfaces of the tendon (figure 1-39). Articular surface partial-thickness tears most commonly affect the anterior aspect of the critical zone of the supraspinatus and are more prevalent than intrasubstance and bursal surface tears. Theoretical basis for the increased frequency of articular surface tears include peculiar histological and biomechanical properties. The bursal side layers are primarily composed of tendon bundles, which are resistant to rupture and may elongate under tensile load, whereas articular side layers are composed of a complex of tendon fibers, ligaments, and joint capsule, which is more susceptible to tear.176
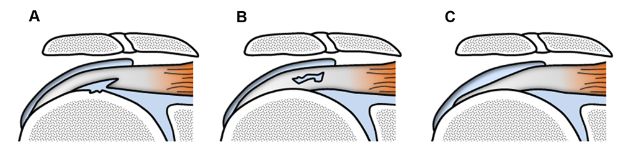
Figure 1-39. Different types of partial-thickness tears. Schematic drawing of a coronal view of the shoulder shows different types of partial-thickness tears. [A] Articular surface. [B] Intrasubstance. [C] Bursal surface. Note bone irregularities commonly observed in articular surface tears.
US is as accurate as MRI to detect partial-thickness tears. In addition, although MR arthrography is currently considered the gold standard for imaging diagnosis, US may be most cost-effective because of its lower cost.177 Management of partial-thickness tears is conservative and surgery typically reserved for refractory symptoms.178
Articular Surface Partial-Thickness Tears
An articular surface partial-thickness tear typically appears as a mixed hyper-hypoechoic defect of the tendon adjacent to the greater tuberosity (figure 1-40). The bursal surface remains intact and shows normal convexity because there is no global volume loss. The terms rim-rent and PASTA (Partial Articular surface Supraspinatus Tendon Avulsion) are commonly used to describe these lesions. As previously mentioned, erosive calcific tendinopathy may simulate rim-rent tears and should always be included in the differential diagnosis (see figure 1-37). Another important differential diagnosis is anisotropy, as both conditions tend to affect articular surface fibers. However, hypoechogenicity caused by anisotropy is artifactual and can be differentiated from a true abnormality because it produces a more homogeneous hypoechoic appearance that can be minimized when the insonating beam is perpendicular to the tendon fibers (figure 1-41). The finding of a cortical irregularity adjacent to the hypoechoic focus is also helpful for differentiation purposes since it is a sensitive sign of an articular surface partial-thickness tear that is not found in anisotropy (video 1-10). These cortical irregularities are believed to result from muscle traction or exposure of the bone previously protected by tendon fibers to the synovial fluid.179 When a focal hypoechoic or anechoic tendon abnormality is seen adjacent to a cortical irregularity, it is important to determine whether the defect communicates with the glenohumeral joint space, representing an articular-surface tear, or is only in contact with the greater tuberosity, representing an intrasubstance tear. Doppler evaluation typically reveals increased vascularity as a normal reparative response to recent small tears, though it is usually unremarkable in chronic or larger tears (figure 1-42).156
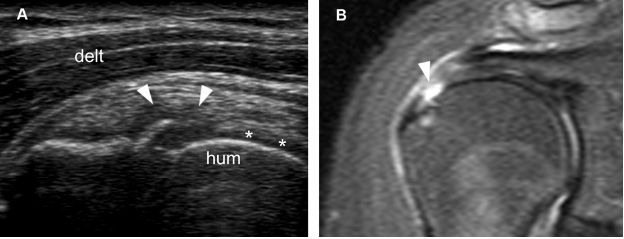
Figure 1-40. Articular surface partial-thickness tear of the supraspinatus tendon. [A] Long-axis 12-5 MHz US image shows a mixed hyper-hypoechoic defect of the tendon (arrowheads). There is also a cortical bone irregularity at the point where the echogenic stria meets the humeral head. In order to correctly diagnose the intra-articular extension of the tear, it is critical to depict contact between the tendon defect and the hyaline cartilage (asterisks). [B] Corresponding coronal oblique STIR MRI confirms the sonographic finding (arrowhead). Hum= humerus. Delt= deltoid.
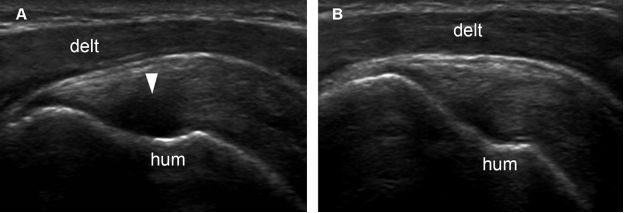
Figure 1-41. Supraspinatus tendon anisotropy. [A] Long-axis 12-5 MHz US image shows artifactual hypoechogenicity where the distal tendon fibers curve downward to insert on the greater tuberosity and are not perpendicular do sonographic beam (arrowhead). [B] With repositioning of the probe, tendon fibers become perpendicular to sonographic beam and show normal echogenicity. Hum= humerus. Delt= deltoid.
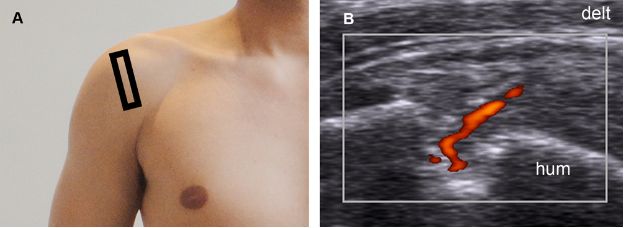
Figure 1-42. Partial-thickness tear of the supraspinatus tendon. [A] Positioning of the probe. [B] Corresponding 12-5 MHz power Doppler US image shows increased vascularity as a normal response to a partial-thickness tear. Hum= humerus. Delt= deltoid.
Articular surface partial-thickness tears affecting the subscapularis, infraspinatus (figure 1-43), and teres minor tendons are relatively rare when compared to supraspinatus tears. Partial tears of the subscapularis tendon are mostly traumatic, while tears of the supraspinatus (video 1-11) and infraspinatus are usually degenerative. Articular surface partial-thickness tears of the infraspinatus have also been described in the setting of internal impingement (see section 2.1.3). In such cases, a labral tear is commonly associated and should be specifically addressed by MRI or MR arthrography. In the posterior aspect of the humerus, care should be taken not to confuse the normal bare area devoid of cartilage with a cortical irregularity secondary to a tear of infraspinatus or teres minor tendons (see figure 6-5).
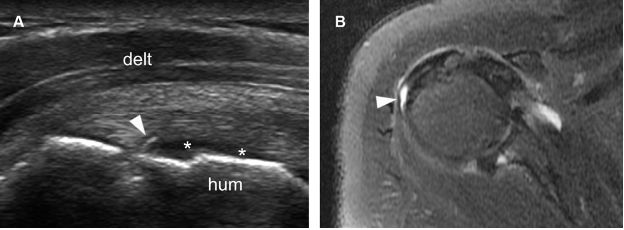
Figure 1-43. Articular surface partial-thickness tear of the infraspinatus tendon. [A] Long-axis 12-5 MHz US image shows a small mixed hyper-hypoechoic defect of the tendon (arrowhead). There is also a cortical bone irregularity at the point where the echogenic stria meets the humeral head and contact between the tendon defect and the hyaline cartilage (asterisks). [B] Corresponding axial STIR MRI confirms sonographic finding (arrowhead). Hum= humerus. Delt= deltoid.
Bursal Surface Partial-Thickness Tears
Because of the superficial location, tendon thinning and volume loss are typical features of bursal surface partial-thickness tears. At US, these tears are depicted as flattening of the bursal surface and loss of convexity of the affected tendon (figure 1-44; video 1-12). These findings are more conspicuous when there is simultaneous fluid distension of the subacromial-subdeltoid bursa (figure 1-45). Dynamic evaluation during graded compression (video 1-13) and both internal and external rotation of the shoulder should be liberally used to differentiate a bursal surface partial-thickness tear from the commoner full-thickness tear (figure 1-46). Some bursal surface tears may extend to the greater tuberosity, but are still considered a partial-thickness tear as long as the articular surface tendon fibers remain intact. In such cases, cortical irregularities very similar to those observed in articular surface tears may result from chronic exposure of the bone to the synovial fluid present in subacromial-subdeltoid bursa.
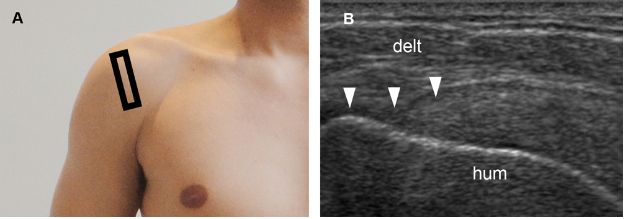
Figure 1-44. Bursal surface partial-thickness tear of the supraspinatus tendon. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows loss of convexity of the supraspinatus tendon (arrowheads). Hum= humerus. Delt= deltoid.
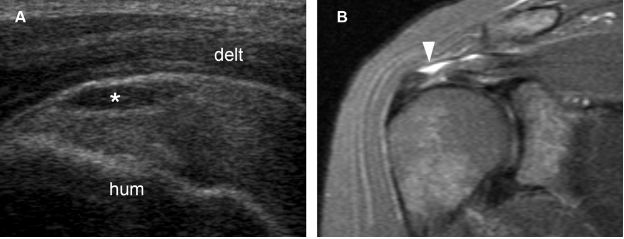
Figure 1-45. Bursal surface partial-thickness tear of the supraspinatus tendon. [A] Long-axis 12-5 MHz US image shows fluid distension of the subacromial-subdeltoid bursa (asterisk) delineating a bursal surface partial-thickness tear of the supraspinatus tendon. [B] Corresponding coronal oblique STIR MRI confirms sonographic finding (arrowhead). Hum= humerus. Delt= deltoid.
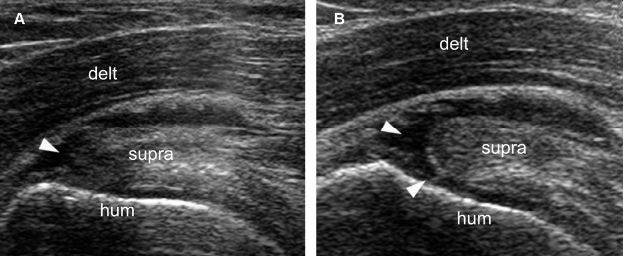
Figure 1-46. Dynamic evaluation of supraspinatus tendon tears. [A] Long-axis 12-5 MHz US image obtained during internal rotation of the shoulder shows a tendon defect consistent with bursal surface partial-thickness tear (arrowhead). [B] Long-axis 12-5 MHz US image obtained during external rotation of the shoulder better delineate the tendon defect that corresponds to a full-thickness tear (arrowheads). Hum= humerus. Delt= deltoid. Supra= supraspinatus.
Intrasubstance Partial-Thickness Tears
Intrasubstance partial tears do not communicate with neither the subacromial-subdeltoid bursa nor the glenohumeral joint. These tears may be located within the tendon substance (figure 1-47) or in contact with the greater tuberosity (figure 1-48). In the latter situation, cortical irregularities secondary to muscle traction are often seen, but volume loss and flattening of the bursal surface of the affected tendon are not typical. Isolated intrasubstance tears not affecting the supraspinatus tendon are exceedingly rare (video 1-14). On a microscopic level, tendon degeneration is characterized by microtears that coalesce to form macroscopic tears, and it may be difficult to arbitrarily differentiate intrasubstance partial-thickness tears from severe tendinopathy.
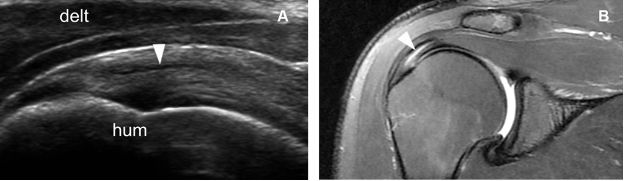
Figure 1-47. Intrasubstance partial-thickness tear of the supraspinatus tendon. [A] Long–axis 12-5 MHz US image shows a discrete intrasubstance tendon defect (arrowhead) surrounded by intact fibers. [B] Corresponding coronal oblique fat-suppressed T2-weighted MRI confirms sonographic finding. Hum= humerus. Delt= deltoid.
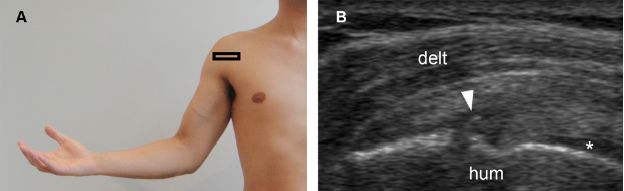
Figure 1-48. Intrasubstance partial-thickness tear of the subscapularis tendon. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a mixed hyper-hypoechoic defect of the tendon (arrowhead) and cortical bone irregularity at the point where the echogenic stria meets the humeral head. Note also intact articular side fibers interposed between the tendon defect and the hyaline cartilage (asterisk). Hum= humerus. Delt= deltoid.
7.3.1. Grading of Partial-Thickness Tears
Grading of partial-thickness tears is much like debating over the war on terror: (1) we know we have a problem, (2) we do not have a consensual solution, (3) everyone has an opinion, and (4) we can make the situation worse. Although different classification schemes are described,180-183 we consider practical to describe only the vertical component of the tear relative to tendon thickness as greater, equal, or lesser than 50%. On daily basis, we describe tears in the 40-60% range as 50%. The horizontal component of a tear has not received much attention from orthopaedic surgeons and its description is considered optional.40,150,184,185 In order not to make the situation worse, the vertical component should not be expressed in absolute terms using submillimetric scale nor in relative terms using fractions of percentage because both situations create an artificial environment for comparison in follow-up (figure 1-49). The reason we choose 50% as a cut-off value refers to surgical planning: debridement is usually undertaken if bursal- or articular-surface tears involve less than 50% of tendon thickness, while repair is generally indicated for larger lesions.186 Evidence to support this approach comes from a cadaveric study that demonstrated consistent increases in supraspinatus strain with articular surface tears involving 50% and 75% of tendon thickness, but not 25%.188 In the same study, tendon repair returned supraspinatus strain almost to the intact levels.187 A partial-thickness tear that involves more than 50% of tendon thickness may be compressible and simulate a full-thickness tear. However, misdiagnosing a high-grade partial-thickness tear for a small full-thickness tear is not clinically relevant because they are often treated as if they were a full-thickness tear.188
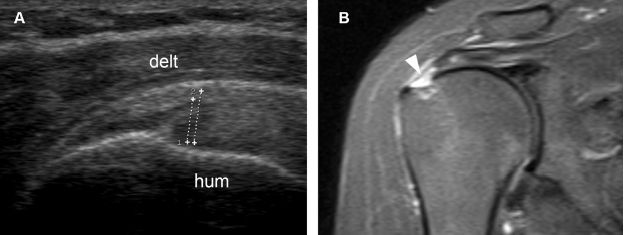
Figure 1-49. Grading a partial-thickness tear of the supraspinatus tendon. [A] Long-axis 12-5 MHz US image shows the vertical component of the tear affecting more than 50% of tendon thickness (cursors). [B] Corresponding coronal oblique STIR MRI confirms sonographic finding (arrowhead). Hum= humerus. Delt= deltoid.
7.3.2 Follow-up of Partial-Thickness Tears
Partial-thickness tears are permanent lesions that do not disappear after successful treatment. Follow-up studies are best reserved for clinically refractory patients at risk for tear progression and should be avoided in the absence of symptoms because informing patient that he still has a lesion may cause symptoms to recur (figure 1-50).
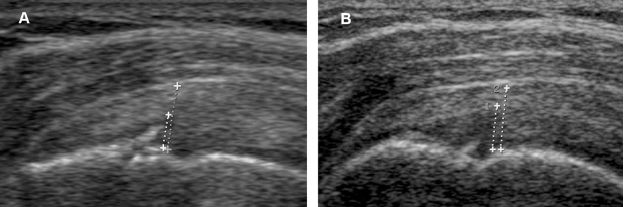
Figure 1-50. Follow-up of a supraspinatus partial-thickness tear. [A] Baseline long-axis 12-5 MHz US image shows the vertical component of the tear affecting 50% of tendon thickness (cursors) [B] Follow-up US obtained ninety days later because of refractory symptoms shows progression of the tear.
7.4. Full-Thickness Tears
Full-thickness tears mostly represent the end result of degenerative changes and chronic partial-thickness tears. Traumatic tears involving otherwise healthy tendons are far less common. The prevalence increases with age, though many are asymptomatic or cause minimal functional disability.
US is an accurate method for diagnosis with reported sensitivity and specificity similar to MRI and estimated at 85% and 86%, respectively.177 Although MR arthrography is considered the gold standard of imaging, the improvement in sensitivity and specificity is in the 7-8% range when compared to US, and it comes at a higher cost.
The primary sonographic finding of full-thickness tears is volume loss and focal tendon nonvisualization. In recent tears, the space left by the missing tendon is filled in by fluid (figure 1-51; video 1-15). Most full-thickness tears occur in the distal enthesis of the supraspinatus, but some may arise more proximally and leave a distal tendon stump attached to the greater tuberosity (figure 1-52; video 1-16). In long-standing tears, fluid is usually absent and the gap filled in by fibrous tissue and proliferating synovium (figure 1-53). Graded compression must be liberally used to depict full-thickness tears because complex fluid, fibrous tissue, and proliferating synovium may all mimic a heterogeneous but intact tendon on static images (video 1-17); dynamic compression induce shift of these material from the tear and allows for accurate diagnosis (figure 1-54; video 1-18). The sonographic appearance of larger chronic tears is more intuitive, since retracted tendon permits abnormal close apposition of the deltoid muscle over the greater tuberosity (figure 1-55).
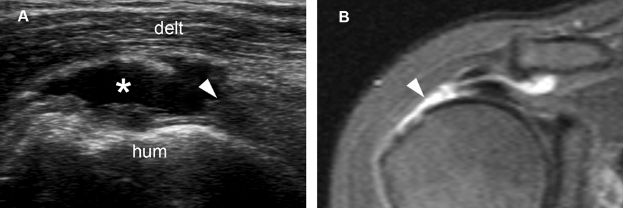
Figure 1-51. Full-thickness tear of the supraspinatus tendon. [A] Long-axis 12-5 MHz US image shows a fluid-filled full-thickness tear of the supraspinatus (asterisk) and proximal tendon retraction (arrowhead). [B] Corresponding coronal oblique STIR MRI confirms sonographic findings (arrowhead). Hum= humerus. Delt= deltoid.
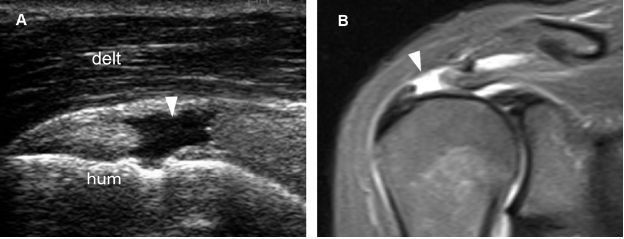
Figure 1-52. Full-thickness tear of the supraspinatus tendon. [A] Long-axis 12-5 MHz US image shows a proximal fluid-filled full-thickness tear of the supraspinatus tendon (arrowhead). [B] Corresponding coronal oblique STIR MRI confirms sonographic finding (arrowhead). Resorption of the distal tendon stump may occur in the long run if these proximal lesions are not operated on. Hum= humerus. Delt= deltoid.
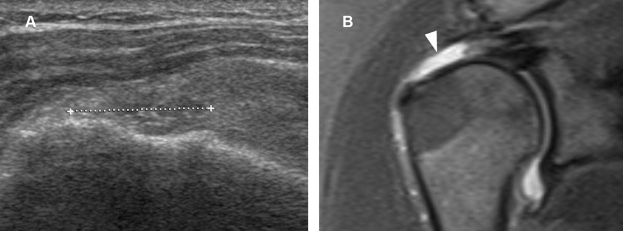
Figure 1-53. Full-thickness tear of the supraspinatus tendon. [A] Long-axis 12-5 MHz US image shows a full-thickness tear of the supraspinatus tendon filled in by fibrous tissue and proliferating synovium (cursors). [B] Corresponding coronal oblique STIR MRI confirms the full-thickness tear (arrowhead).
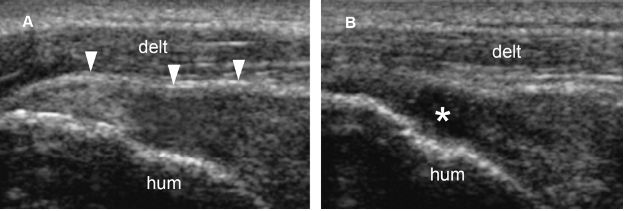
Figure 1-54. Full-thickness tear of the supraspinatus tendon. [A] Long-axis 12-5 MHz US image shows loss of convexity of the tendon (arrowheads). [B] Graded compression with the probe induces shift of the echogenic material from the tear and clearly demonstrates a full-thickness tear (asterisk). Hum= humerus. Delt= deltoid.
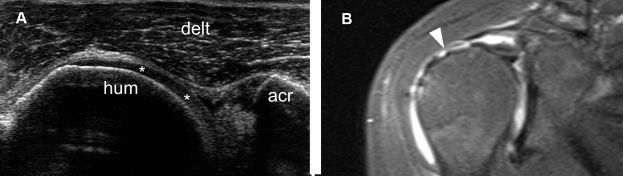
Figure 1-55. Full-thickness tear of the supraspinatus tendon. [A] Long-axis 12-5 MHz US image shows retracted supraspinatus tendon permitting abnormal close apposition of the deltoid (delt) over the hyaline cartilage (asterisks). [B] Corresponding coronal oblique STIR MRI confirms the full-thickness tear of the supraspinatus tendon (arrowhead). Hum= humerus. Acr= acromion.
A common cause of false-negative US is incomplete evaluation of the most anteromedial aspect of the supraspinatus, near to the rotator interval, in a region also known as the cemetery of tears because tendon tears figuratively pretend they are dead and accumulate in this location to be left undisturbed (video 1-19). Hence, it is important that the entire extent of the supraspinatus be completely evaluated, and this can be assured by moving the probe anteriorly on the greater tuberosity until the intra-articular portion of the long head of the biceps brachii is seen.
Full-thickness tears may also affect the subscapularis, infraspinatus (figure 1-56), and teres minor tendons, in these two latter usually secondary to posterior extension of a supraspinatus tear.189 Dynamic evaluation during internal and external rotation of the shoulder is useful to make full-thickness tears of the infraspinatus tendon more conspicuous (figure 1-57; videos 1-20 and 1-21) and help to depict both acute (video 1-22) and chronic (video 1-23) tears of the subscapularis tendon.
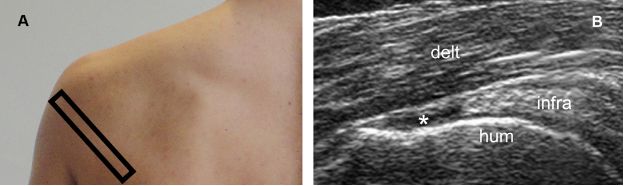
Figure 1-56. Full-thickness tear of the infraspinatus tendon. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a fluid-filled full-thickness tear of the infraspinatus tendon (asterisk). Infra= infraspinatus. Hum= humerus. Delt= deltoid.
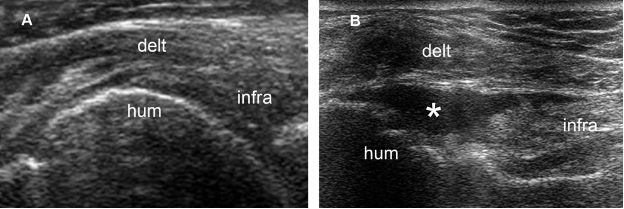
Figure 1-57. Full-thickness tear of the infraspinatus tendon. [A] Long-axis 12-5 MHz US image obtained during internal rotation of the shoulder shows a heterogeneous but apparently intact infraspinatus tendon. [B] Long-axis 12-5 MHz US image obtained during external rotation of the shoulder depicts a fluid-filled full-thickness tear (asterisk). Infra= infraspinatus. Hum= humerus. Delt= deltoid.
In addition to volume loss and focal tendon nonvisualization of the tendon, several secondary findings of full-thickness tears are described. From these, the most significant is the coexistence of fluid distension of the subacromial-subdeltoid bursa and the glenohumeral joint recesses (figure 1-58). Another secondary finding is the presence of a linear bright reflection at the superficial margin of the articular cartilage of the humeral head, caused by reduced sound attenuation through a tear, commonly referred to as the cartilage interface sign. However, this finding is not specific for full-thickness tears and only depicted when US beam is perpendicular to the hyaline cartilage (figure 1-59). A third secondary finding is the identification of an intramuscular ganglion, since a defect in the surface of a tendon may allow fluid to dissect along the fibers and collect within the muscle (figure 1-60).190 A fourth and rare secondary finding is the geyser sign, related to communication between the subacromial-subdeltoid bursa and the glenohumeral and acromioclavicular joints (figure 1-61).191 There are many other secondary findings described in the literature for the enthusiasts but one should keep in mind that the sonographic diagnosis of a full-thickness tear requires volume loss and focal tendon nonvisualization, and none of these secondary findings is critical nor should be used as a sole criterion for diagnosis.
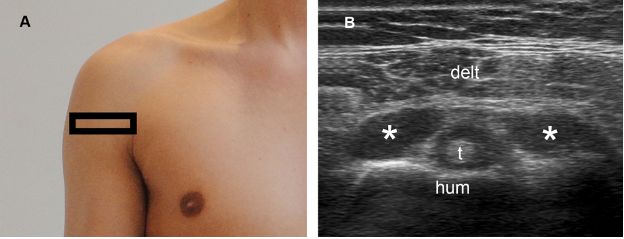
Figure 1-58. Secondary finding of full-thickness tears. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows concomitant fluid distension of the subacromial-subdeltoid bursa (asterisks) and the long head of the biceps brachii tendon (t) sheath. Hum= humerus. Delt= deltoid.
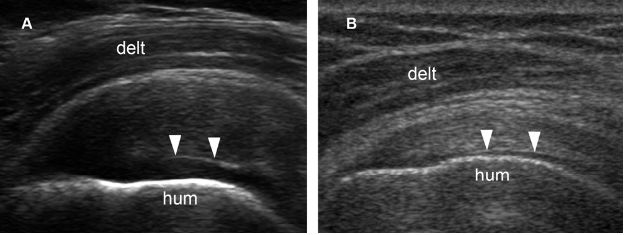
Figure 1-59. Cartilage interface sign. [A] Long-axis 12-5 MHz US image shows the cartilage interface sign (arrowheads) associated to supraspinatus tendinopathy. [B] Long-axis 12-5 MHz US image show the cartilage interface sign (arrowheads) associated to a normal supraspinatus tendon. The cartilage interface sign is nonspecific and can be found in the full spectrum of rotator cuff imaging, from normal tendons to complete tears. In order to increase the specificity for full-thickness tears, some authors consider this finding positive only when there is a more pronounced brightly echogenic interface. Hum= humerus. Delt= deltoid.
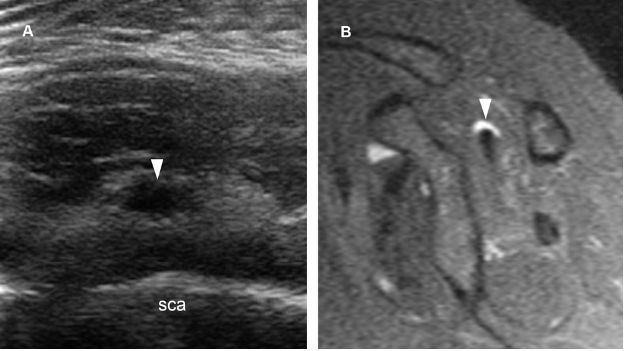
Figure 1-60. Intramuscular ganglion. [A] Short-axis 12-5 MHz US image obtained from a dorsal approach shows a supraspinatus intramuscular ganglion (arrowhead). [B] Corresponding sagittal STIR MRI confirms sonographic finding (arrowhead). Sca= scapula.
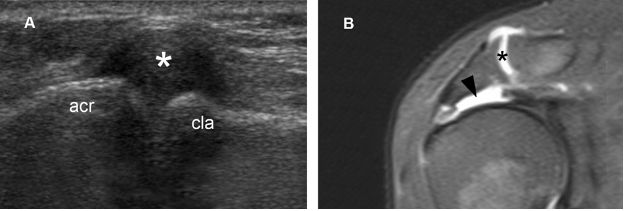
Figure 1-61. Geyser sign. [A] Long-axis 12-5 MHz US image shows distended acromioclavicular joint capsule (asterisk). [B] Corresponding coronal oblique STIR MRI confirms sonographic finding (black asterisk) and also shows the related supraspinatus full-thickness tear (black arrowhead). The geyser sign was first described on shoulder arthrography and resulted from leakage of contrast medium from the glenohumeral joint into the subacromial-subdeltoid bursa to outline the acromioclavicular joint like a geyser. Acr= acromion. Cla= clavicle.
7.4.1. Grading of Full-Thickness Tears
Essential information for treatment and prognosis includes the degree of tendon retraction, the cuff muscle status, and the acromiohumeral distance.
Various schemes have been used to classify the degree of tendon retraction, but each methodology has limitations. Bateman classified rotator cuff tears as either small (<1 cm), medium (1-3 cm), large (3-5 cm), or massive (>5 cm).192 Alternatively, Patte proposed a classification based on tendon retraction in the coronal plane.193 Tauro proposed an index calculated by multiplying the mediolateral by the anteroposterior dimension of the tear.194 From a practical standpoint, it may be useful to simply report the greatest dimension of the tear in two orthogonal planes, so the referring physician can use the classification system he likes the most. Supraspinatus tears greater than 3 cm may be problematic to measure because the proximal tendon stump is usually hidden under the acromion.
A full-thickness tear that involves the entire width of a tendon may be termed a complete tear (figure 1-62). However, since distal fibers of the supraspinatus interdigitate with the infraspinatus near to the greater tuberosity, it may be difficult to accurately distinguish complete from incomplete tears when assessing these tendons. In such cases, the key point is not to categorize the lesion as complete or incomplete, but to accurately inform tendon retraction. The subscapularis tendon is a whole different story because it is clearly differentiated from the adjacent tendons. Hence, besides inform tendon retraction, full-thickness tears of the subscapularis tendon are ideally also described as complete (figure 1-63) or incomplete (figure 1-64).195
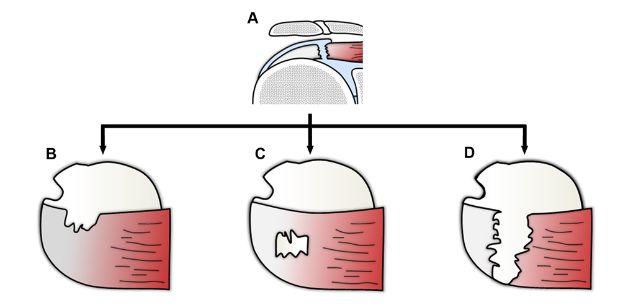
Figure 1-62. Different morphologies of full-thickness tears of the supraspinatus. Schematic drawing of a [A] coronal and [B,C,D] superior view of the shoulder showing different morphologies of full-thickness tears.
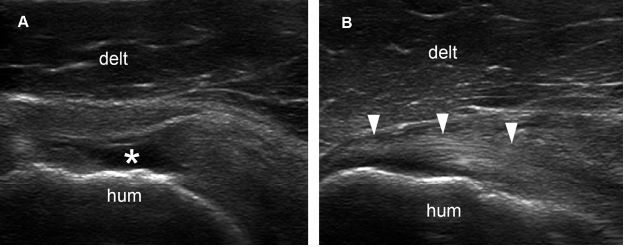
Figure 1-63. Incomplete (full-thickness/partial-width) tear of the subscapularis tendon. [A] Long-axis 12-5 MHz US image shows a fluid-filled full-thickness tear of the superior insertion of the subscapularis tendon (asterisk). [B] Long-axis 12-5 MHz US image obtained just caudal to A shows intact inferior insertion (arrowheads). Hum= humerus. Delt= deltoid.
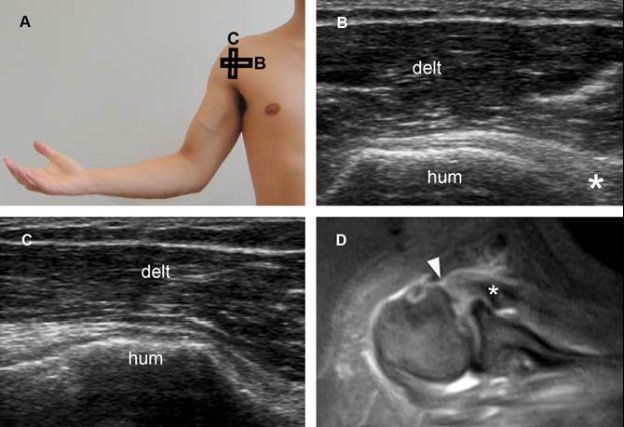
Figure 1-64. Complete (full-thickness/full-width) tear of the subscapularis tendon. [A] Positioning of the probe. [B] Corresponding long-axis 12-5 MHz US image shows a full-thickness tear of the subscapularis. Retracted tendon (asterisk) permits abnormal close apposition of the deltoid (delt) and humeral head (hum). [C] Corresponding short-axis 12-5 MHz US image shows full-with tear, involving both superior and inferior insertion of the tendon. [D] Corresponding axial STIR MRI confirms tendon retraction (asterisk) and a full-thickness/full-width tear of the subscapularis tendon (arrowhead).
Rotator cuff muscle status should be routinely assessed as fatty infiltration, muscle atrophy, and fatty atrophy constitute chronic findings that are considered bad prognostic markers for both surgical and conservative treatment.196-198 These terms are often loosely applied and used inter-changeably to describe increased echogenicity of muscular tissue at US, though muscle atrophy or fatty atrophy are usually preferred when there is a gross decrease of muscle bulk (figure 1-65). Abnormal muscle echogenicity is assessed subjectively by comparison with the trapezius. As a rule, fat replacement secondary to tendon tears represents the main cause of hyperechoic cuff muscles relative to trapezius, while trauma, denervation, and myositis are less probable diagnoses. With increased muscle echogenicity, the sharp contrast between the hyperechoic perimysium and hypoechoic muscle bundles becomes less distinct, leading first to the effacement and then to the disappearance of the pennate pattern.199 Infraspinatus muscle atrophy may develop after rotator cuff tears with an intact infraspinatus tendon200 and the same applies to the subscapularis (figure 1-66).201 Isolated or more pronounced atrophy of the infraspinatus in the setting of a tear limited to the supraspinatus may be related to compromised supraescapular nerve from altered biomechanics.202 Fatty infiltration secondary to tendon tears is not a true degenerative process, but rather a necessary rearrangement of the tissue after a change in the pennation angle caused by musculotendinous retraction.203 The successful treatment of tendon tears often prevents further atrophy and fatty infiltration to develop, but does not significantly reverse already installed abnormalities.
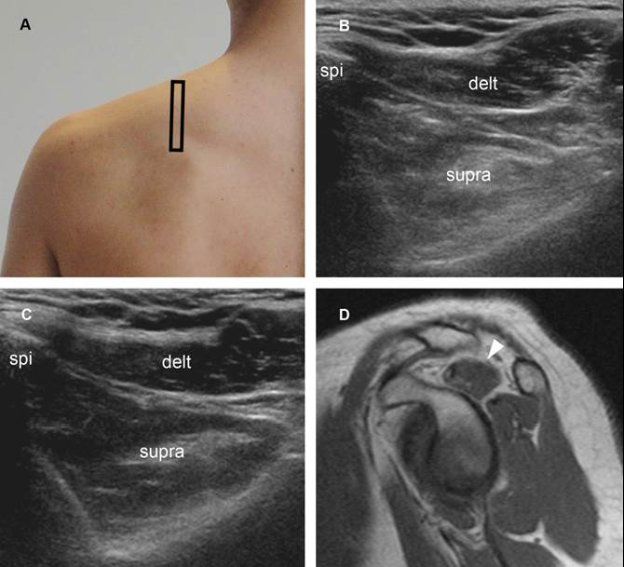
Figure 1-65. Supraspinatus muscle atrophy. [A] Positioning of the probe. [B] Corresponding short-axis 12-5 MHz US image shows reduced volume and increased echogenicity of the supraspinatus muscle (supra) in symptomatic side. [C] Contralateral comparative 12-5 MHz US image shows normal supraspinatus muscle in asymptomatic side. [D] Corresponding sagittal T1-weighted MRI confirms supraspinatus muscle atrophy in symptomatic side (arrowhead). Delt= deltoid. Spi= spine of scapula.
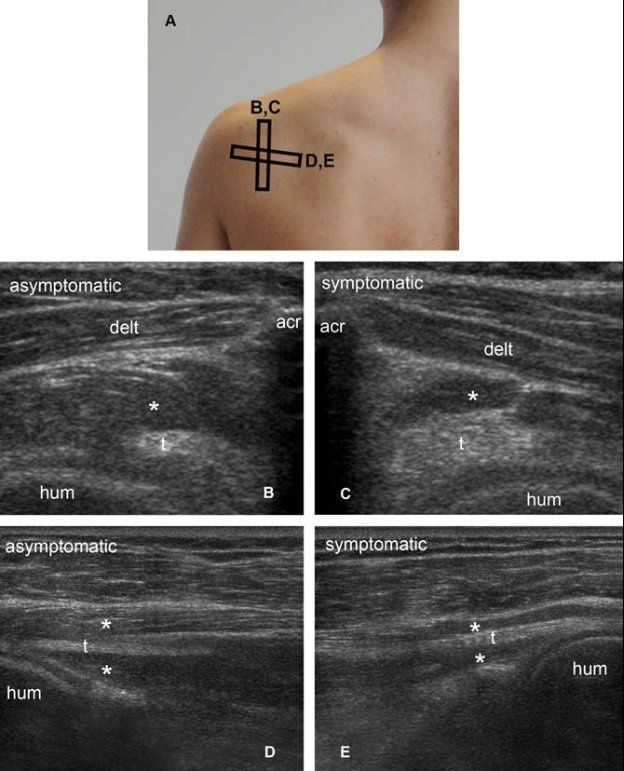
Figure 1-66. Infraspinatus muscle atrophy. [A] Positioning of the probe. [B] Corresponding short-axis 12-5 MHz US image shows normal infraspinatus muscle (asterisk) and tendon (t) in asymptomatic side. [C] Corresponding short-axis 12-5 MHz US image shows loss of muscle mass (asterisks) compared to contralateral asymptomatic side. [D] Corresponding long-axis 12-5 MHz US image shows normal infraspinatus muscle (asterisk) and tendon (t) in asymptomatic side. The pennate pattern is depicted as multiple well-defined linear and parallel hyperechoic tissue composed of the fibroadipose septa of the perimysium surrounding hypoechoic muscle bundles. [E] Corresponding long-axis 12-5 MHz US image shows reduced muscle mass (asterisks) and loss of pennate pattern with poor visibility of the hyperechoic perimysium in symptomatic side. Acr= acromion. Hum= humerus. Delt= deltoid.
It is important to recognize that hyperechoic cuff muscles are not synonymous with rotator cuff tear as paralabral cysts may cause suprascapular nerve entrapment that denervates both supraspinatus and infraspinatus when located in the supraescapular notch (figure 1-67), or generate isolated denervation of the infraspinatus when located in the spinoglenoid notch (figure 1-68). Teres minor atrophy may be observed in up to 3% of the shoulders and is often asymptomatic,204 though it occasionally relates to quadrilateral space syndrome (figure 1-69).
Hyperechoic cuff muscles are not necessarily pathological and may develop to a lesser extend as a result of aging, in a process known as sarcopenia. Sarcopenia diffusely affects different muscles of the shoulder, and the use of the trapezius and contralateral cuff muscles as a reference standard may help to correctly identify expected senile fat replacement and differentiate it from pathological changes.
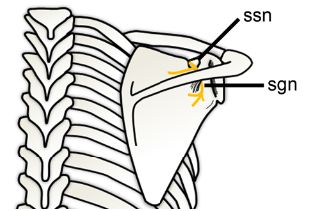
Figure 1-67. Suprascapular nerve entrapment. Schematic drawing of a posterior view of the shoulder shows the suprascapular nerve (yellow). The suprascapular nerve is motor and originates from C5 and C6 nerve roots or upper trunk of the brachial plexus. It passes through the suprascapular notch (ssn) into the supraspinatus fossa to supply the supraspinatus muscle. The nerve continues around the border of the spine of the scapula to the spinoglenoid notch (sgn) to supply the infraspinatus.
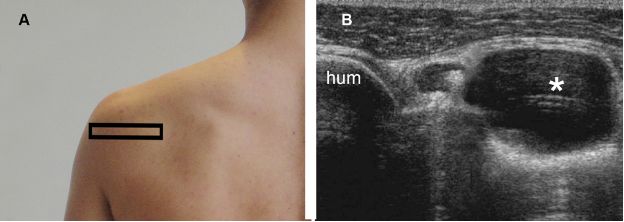
Figure 1-68. Paralabral cyst at the spinoglenoid notch. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a paralabral cyst (asterisk) at the spinoglenoid notch. Hum= humerus.
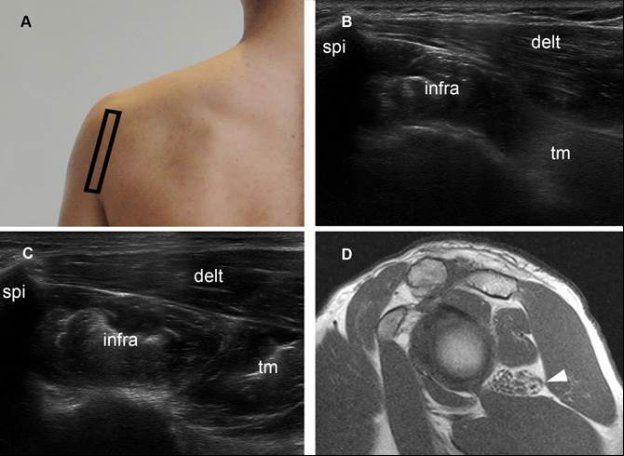
Figure 1-69. Quadrilateral space syndrome. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US dual-image shows reduced volume and increased echogenicity of the teres minor muscle ™. Note normal size and echogenicity of both infraspinatus (infra) and deltoid (delt) muscles. [C] Contralateral comparative 12-5 MHz US shows normal muscles in asymptomatic side. [D] Corresponding sagittal T1-weighted MRI confirms isolated atrophy of the teres minor muscle (arrowhead). The quadrilateral space is traversed by the axillary nerve and bounded superiorly by the teres minor, inferiorly by the teres major, medially by the long head of the triceps, and laterally by the humeral neck.210 Neural compression by fibrous bands related to microtrauma, such as a result of repeated overhead movements, is the most common cause of the syndrome.211 Other causes include cysts, hematomas, and soft-tissue tumors. At US, the teres minor or deltoid muscle, or both, may appear hyperechoic and small as a result of atrophy.212 Spi= spine of scapula.
Finally, acromiohumeral distance also provides important prognostic information and is defined as the shortest distance between the acromion and the humerus (figure 1-70).205 When measured on standard anteroposterior radiographs, an acromiohumeral distance of 7 mm or less suggest a large cuff tear, correlates with rotator cuff muscle atrophy, and predicts poor surgical outcome (figure 1-71).206 The unopposed action of the deltoid muscle actively pulling the humerus superiorly in the absence of the supraspinatus due to tear is the basis for the decreased acromiohumeral distance.207
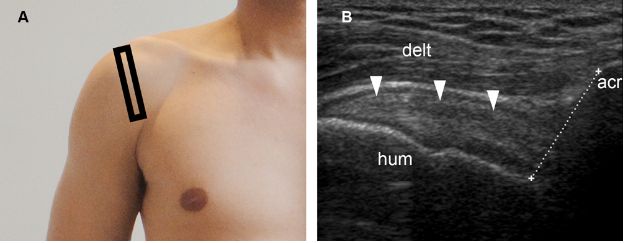
Figure 1-70. Normal acromiohumeral distance. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows normal supraspinatus tendon (arrowheads) and acromiohumeral distance (cursors). Acr= acromion. Hum= humerus. Delt= deltoid.
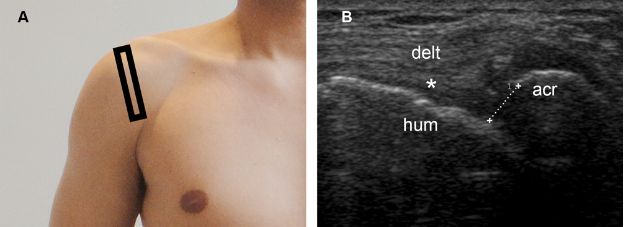
Figure 1-71. Reduced acromiohumeral distance. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a full-thickness tear of the supraspinatus tendon (asterisk) permitting abnormal close apposition of the deltoid (delt) over the humeral head (hum). Note also reduced acromiohumeral distance (cursors). Acr= acromion.
7.4.2. Follow-up of Full-Thickness Tears
As is the case with the partial-thickness tears, full-thickness tears are permanent lesions that do not disappear over time. The role of follow-up studies is to access prognostic information for optimal treatment planning, including location of the tear, degree of tendon retraction, cuff muscle status, and acromiohumeral distance.
Treatment is best decided on a case-by-case analysis, and multiple factors require consideration.208 Acute traumatic full-thickness tears are probably best repaired as soon as possible, in order to avoid tendon resorption and muscle atrophy.209 In the remaining cases, surgical treatment is typically reserved for patients refractory to conservative treatment. Asymptomatic tears are not usually considered candidates for surgery.
Cuff tear arthropathy may be detected during follow-up and occurs as the end result of chronic full-thickness tears (see section 2.2.2 in chapter 5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree