Robert N. Gibson, Thomas Sutherland
The Biliary System
Biliary Anatomy
The intrahepatic pattern of bile duct branching is best described according to the system of Healey and Schroy, to which can be applied the Couinaud system for numbering segments. The typical pattern and its variations are shown in Figs. 32-1 and 32-2. The confluence of the bile ducts is a bifurcation in about 60% of individuals and a trifurcation in about 12% (Fig. 32-3).1,2 A right sectoral duct crosses to the left to join the left hepatic duct in 28% of cases (22% right posterior sectoral, 6% right anterior sectoral) (Fig. 32-4).1 Occasionally a right posterior sectoral or segmental duct (more often posterior than anterior) courses inferiorly and either enters the common hepatic duct directly or cystic duct (Fig. 32-5). Other uncommon left branching variations are shown in Figs. 32-2 (E and F).
The cystic duct typically joins the common hepatic duct in the middle third of the extrahepatic bile duct—often referred to as the ‘common duct’ on ultrasound (US) for convenience—which then continues as the common bile duct (CBD). The cystic duct usually joins the right side of the common duct but can pass behind or in front of the common duct to join it from the left. The cystic duct can join the common duct at a very low level, in which case it may be mistaken for the common duct on imaging. Uncommonly it may join a right-sided duct, which is usually a low, aberrant right sectoral or segmental duct (Fig. 32-5). Some of these variations predispose patients to duct injury at cholecystectomy.
Other variations include ducts of Luschka or subvesical ducts, and cystohepatic ducts. There is some confusion over nomenclature but it seems that the terms subvesical duct and duct of Luschka both describe an intrahepatic duct running adjacent to the gallbladder fossa, unaccompanied by a portal vein branch, and emptying into either the right hepatic or common hepatic duct. The term ‘cystohepatic duct’ is probably best reserved for small ducts that drain directly into the gallbladder or cystic duct. The significance of these variants is their proximity to the gallbladder and the potential for injury at cholecystectomy resulting in a bile leak.3
It is important to remember that the anterior position of the left intrahepatic ducts affects the pattern of filling at direct cholangiography. During percutaneous transhepatic cholangiography (PTC) or T-tube cholangiography it may be necessary to roll the patient to the left to ensure good left-duct filling. Conversely the left ducts fill first at endoscopic retrograde cholangiopancreatography (ERCP) with the patient in the prone position. Furthermore, as the patient is usually oblique during ERCP, the left-sided ducts are often projected to the right and may be misinterpreted as being right-sided ducts if there is incomplete filling of intrahepatic ducts.
Gallbladder Anatomical Variants
Agenesis of the gallbladder is extremely rare, with a prevalence of 0.03–0.07%. A double gallbladder occurs in about 0.03%, usually with a shared cystic duct, and the accessory gallbladder is often diseased.
True gallbladder septa are uncommon and when occurring at the fundus form a Phrygian cap. Frequently, an apparent septum is merely gallbladder wall folding, which can vary with patient position.
The gallbladder can be abnormal in position, being retrohepatic, suprahepatic, left-sided or intrahepatic, the latter potentially presenting as a liver abscess if complicated by acute cholecystitis. A number of forms of left-sided gallbladder exist:
Methods of Investigation
Ultrasound
Transabdominal ultrasound is frequently the first imaging technique employed for patients presenting with hepatobiliary-type symptoms as it is more accurate than CT for diagnosing acute biliary disease.4 Imaging is usually performed following a 4-hour fast, allowing the gallbladder to fill and reducing obscuring upper abdominal gas. The wall of a normal non-contracted gallbladder is <3 mm thick and is smooth. Ultrasound allows a dynamic assessment and by moving the patient helps differentiate stones, sludge and polyps. Doppler ultrasound allows assessment of vascularity, while focal gallbladder tenderness can be determined using probe pressure. The normal cystic duct may not be visible; however, the extrahepatic bile duct can be seen as a tubular structure anterior to the portal vein and lacking blood flow on Doppler.
Contrast-enhanced ultrasound (CEUS) using second-generation microbubble agents can be performed at transabdominal, endoscopic and intraoperative ultrasound. CEUS is useful in selected patients: for example, in differentiating sludge from tumour, identifying perforation in cholecystitis and better demonstrating hilar cholangiocarcinoma.
Computed Tomographic Cholangiography
Computed tomographic (CT) cholangiography relies on either oral or intravenous (IV) contrast agents that are excreted preferentially by the liver, to opacify the bile ducts. Most centres now use IV contrast agents for CT cholangiography, so-called CT-intravenous cholangiography (CT-IVC). This technique involves the IV infusion of an agent such as sodium ipodate with helical CT performed about 30 minutes later. Multislice helical CT allows high-resolution imaging and multiplanar reformatting. Prone imaging can be performed after supine imaging if there is substantial intraductal gas or contrast layering.
Sodium iotroxate is safer than older IV biliary agents, with reported complications in 3.5% of patients (3.0% minor, 0.3% moderate and 0.2% severe)5 and an estimated mortality rate of 0.005%.6
Adequate contrast excretion relies on near-normal hepatocyte function, so the technique is of no value in the investigation of jaundice, and usually fails if bilirubin levels are more than about two times normal.
Since CT-IVC relies on the excretion and subsequent passage of a contrast agent, it provides a functional dimension not obtained with conventional magnetic resonance cholangiography (MRC), allowing the direct demonstration of bile leaks, biliary communication with cysts and segmental obstruction.
Magnetic Resonance Cholangiopancreatography
Magnetic resonance cholangiopancreatography (MRCP) has substantially replaced diagnostic PTC and ERCP. It relies on heavily T2-weighted sequences that display stationary water as high signal. Multiplanar thin and thick section acquisitions are obtained using fast spin-echo techniques. Since conventional MRCP is not reliant on contrast excretion it is suitable for jaundiced patients, a clear advantage compared with CT-IVC.
More recently MR has been combined with hepatobiliary contrast agents. These agents, which include mangafodipir trisodium, gadobenate dimeglumine and gadoxetic acid disodium, shorten T1 relaxation, providing positive contrast images on T1-weighted sequences. Imaging is performed at least 30 minutes after IV infusion to allow hepatocyte uptake and biliary excretion. It therefore provides functional as well as anatomical information but, as with CT-IVC, depends on near-normal excretory hepatocyte function.7 Since T1-weighted MR sequences are used it is possible to use near-isotropic three-dimensional gradient echo acquisitions. Contrast-enhanced MR cholangiography using hepatobiliary contrast agents has similar applications to CT-IVC, except that it is not as sensitive as conventional MRCP for the detection of choledocholithiasis.
Diagnostic pitfalls with MRCP include localised signal voids caused by surgical clips, and intraductal gas or blood. Bile flow voids may mimic small stones but the former are centrally placed and have less well-defined margins than stones. Acquisition times are longer for MRCP than CT-IVC and therefore more prone to motion artefacts.
Endoscopic Retrograde Cholangiopancreatography
ERCP provides direct opacification of bile ducts and pancreatic ducts, with success rates of 92–97%, and provides dynamic information during contrast medium introduction and drainage. It allows visual assessment of the duodenum and ampulla of Vater and enables biopsy and brushings, as well as interventional procedures such as sphincterotomy and stone extraction, biliary stenting and biliary stricture dilatation. Complication rates vary depending on the indication for the procedure, the presence of coexisting disease and the experience of the endoscopist, with severe complication rates of 0.9 to 2.3%, and total complication rates of 8.4–11.1%, the most common significant complication being acute pancreatitis.8 The main diagnostic pitfall with ERCP is the underfilling of ducts above a stricture.
Percutaneous Transhepatic Cholangiography
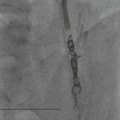
PTC has been substantially replaced by ERCP and MRCP. Its role now is mostly as part of transhepatic biliary intervention. A 22G Chiba needle is used to puncture the right or left intrahepatic ducts from the right flank or, for left ducts, from an epigastric approach. The epigastric approach is used if the left ducts cannot be opacified from the right or as part of a left-lobe approach to biliary drainage. Any coagulation disorder should be reversed prior to the procedure, which is performed with broad-spectrum IV antibiotic cover and conscious sedation or occasionally general anaesthesia.
If the ducts are dilated, the needle is withdrawn gradually with suction applied and when bile is aspirated contrast medium is injected to opacify the biliary tree. If the ducts are not dilated, or if initial passes fail to opacify them, small injections of contrast medium are made as the needle is gradually withdrawn until bile ducts are opacified. Samples should be taken for microbiology and, if malignant obstruction is suspected, cytological examination. Care should be taken to opacify the entire biliary tree, especially in cases of lobar or segmental biliary obstruction. A common pitfall is the failure to fill the left hepatic ducts from a right-sided approach and this should be suspected if no ducts are opacified in the midline. The aspiration of some bile during the procedure reduces the risk of bile leak and endotoxaemia by reducing intraductal pressure.
Success rates are close to 100% if the ducts are substantially dilated and about 75% if they are non-dilated or only slightly dilated, the latter group usually requiring multiple needle passes. US guidance may be used if a particular segment is to be opacified and some operators use it routinely for PTC or percutaneous transhepatic biliary catheterisation. The major complication rate is about 4% and includes haemobilia, bacteraemia and bile leak.9
Intraoperative Cholangiography
Intraoperative cholangiography (IOC) is performed routinely or selectively during cholecystectomy to detect choledocholithiasis, confirm duct stone clearance and delineate anatomy to minimise risk of bile duct injury.
T-Tube Cholangiography
If the CBD has been explored at cholecystectomy a T-tube is usually left in place and cholangiography performed via this tube after about 7 days, prior to its removal. Cholangiography should confirm stone clearance and the free passage of contrast medium into the duodenum. Care must be taken to avoid the injection of air bubbles.
Hepatobiliary Scintigraphy
Hepatobiliary iminodiacetic acid (HIDA) scintigraphy uses a derivative of iminodiacetic acid, a bilirubin analogue, labelled with 99mTc. It is injected intravenously and serial gamma camera images are obtained over 2–4 hours. It relies on near-normal bilirubin levels, although some agents can be excreted with moderate elevations of bilirubin. Serial image acquisitions show accumulation of the isotope in the liver, bile ducts, duodenum, small bowel and gallbladder (providing it is present and the cystic duct is patent).
Endoscopic Ultrasound
Biliary endoscopic ultrasound (EUS) provides high-frequency grey-scale imaging, colour Doppler and contrast-enhanced ultrasound for evaluation of the extrahepatic biliary tree and pancreas. EUS has similar sensitivity and specificity to MRCP in diagnosing causes of biliary obstruction.10 Though relatively invasive, advantages are that it allows direct visualisation of the duodenum, fine-needle aspiration cytology and potentially biliary drainage. More sophisticated and expensive systems of ‘mother-daughter’ probes allow intraductal examination of the CBD, but are not routinely available.
Disorders of the Gallbladder
Gallbladder Stones
The prevalence of gallbladder stones in adults in Western communities is approximately 15%. They are asymptomatic in about 80% but in this group about 15% will develop symptoms over 15 years and they confer a small lifetime risk of gallbladder carcinoma. About 70% of gallbladder stones are solely or predominantly cholesterol in type, with up to 30% being black pigment stones composed mainly of calcium bilirubinate.11
Less than 10% of stones are opaque on plain radiographs, the larger stones showing laminated or peripheral calcification. On CT a minority of gallbladder stones are visible, being hyperdense, hypodense, or of mixed density.
US is the most accurate investigation for the diagnosis of gallbladder stones, which appear as echogenic foci producing acoustic shadows (Fig. 32-6). Stone mobility is frequently identifiable (Fig. 32-7), though is not essential for diagnosis. The sensitivity of US is > 95%.12 False-negative diagnoses are commoner than false-positive ones, and are usually due to small stones in patients in whom there is poor acoustic access to the gallbladder because of obesity or other unfavourable anatomy. False-negative diagnoses are reduced by careful US technique. Small stones are differentiated from small polyps by the demonstration of mobility or the presence of an acoustic shadow.
Non-visualisation of the gallbladder on US can be due to a previous cholecystectomy, non-fasting state, an abnormal gallbladder position, emphysematous cholecystitis, or because the gallbladder is filled with stones. The latter can be recognised by identifying the so-called ‘double-arc shadow’ sign in the gallbladder fossa, consisting of two parallel curved echogenic lines separated by a thin anechoic space with dense acoustic shadowing distal to the deeper echogenic line (Fig. 32-8).
Sludge
Sludge is commonly seen on US and appears as fine, non-shadowing dependent echoes. It is composed of calcium bilirubinate granules, cholesterol crystals and glycoproteins.13 It is more commonly seen in chronic fasting states, critically ill patients, those receiving total parenteral nutrition and in pregnancy. Sludge resolves spontaneously in 50% of patients and gallstones will develop in 5–15%.14
Small stones are difficult to locate within sludge, so careful imaging through sludge is important (Fig. 32-9). Usually sludge layers in a dependent fashion but occasionally it mimics a tumour mass, i.e. ‘tumefactive sludge’. Sludge can usually be differentiated from tumour by its mobility, lack of internal blood flow on Doppler examination, lack of focal gallbladder wall abnormality, or lack of enhancement on CEUS.
Blood (haemobilia) and pus (empyema) may have a similar appearance to sludge and the clinical setting aids in their differentiation. Sludge, blood and pus can also occur in the bile ducts.
Milk of Calcium Bile
Milk of calcium bile, or limy bile, is an uncommon condition in which the gallbladder bile becomes viscous, probably as a result of stasis, and contains a high concentration of calcium bilirubinate. On US it causes diffuse echoes, similar to sludge, but is more echogenic (Fig. 32-10A) with a tendency to layer out and produce an acoustic shadow. On CT (Fig. 32-10B) and, occasionally, on plain radiographs it is visible as layering high-density material.
Cholecystitis
Acute Calculous Cholecystitis
US is the best initial imaging investigation in patients with suspected acute cholecystitis, which, in 90–95% of cases, is due to gallstones (acute calculous cholecystitis). The positive predictive values of stones combined with either tenderness localised to the gallbladder (positive sonographic Murphy’s sign), or the presence of a gallbladder wall thickness of >3 mm, are 92 and 95%, respectively (Fig. 32-11). The negative predictive value of the absence of gallbladder stones and a negative sonographic Murphy’s sign is 95%. US can be definitive in about 80% of cases.15 Gallstone(s) may be impacted in the gallbladder neck and this region must be carefully examined. Other US signs are gallbladder distension (diameter >5 cm), pericholecystic fluid, gallbladder wall striations and, occasionally, wall hyperaemia on Doppler examination. Fine echoes within the gallbladder may be due to sludge or pus (gallbladder empyema). If liver function tests suggest duct obstruction, a careful evaluation of the CBD should be made for choledocholithiasis.
CT is less accurate than US for acute cholecystitis, but is widely used to evaluate patients with acute abdominal pain. The CT findings in acute cholecystitis include gallbladder wall thickening, subserosal oedema, gallbladder distension, high-density bile, pericholecystic fluid and inflammatory stranding in pericholecystic fat (Fig. 32-12). Gallstones are identifiable in the minority. Gallbladder wall enhancement is variable and not a reliable predictor of cholecystitis since normal gallbladders can show wall enhancement. Transient pericholecystic liver rim enhancement may be seen.16
Hepatobiliary scintigraphy has a high diagnostic accuracy for acute cholecystitis. A positive result is non-visualisation of the gallbladder, which results from cystic duct obstruction. Although its accuracy is similar to US, it is more time consuming and does not allow assessment of related organs. It can be helpful, however, when diagnostic uncertainty remains after US.
Gallbladder wall thickening may result from many causes other than cholecystitis. These include non-fasting state, generalised oedematous states, hepatitis, pancreatitis, gallbladder wall varices, adenomyomatosis and carcinoma, though the latter two usually cause focal rather than diffuse thickening.
Gangrenous Cholecystitis
This condition is suggested on US by pronounced irregularity or asymmetrical thickening of the gallbladder wall, internal membranous echoes resulting from sloughed mucosa and pericholecystic fluid. The clinical findings, paradoxically, may diminish with progression to gangrenous change. CT signs suggesting gangrenous cholecystitis are gas in the wall or lumen, discontinuous and/or irregular mucosal enhancement (Fig. 32-13), internal membranes and pericholecystic abscess. Of these, interrupted wall enhancement is the most sensitive sign (70.6%) and is highly specific (100%).17
Gallbladder perforation, occurs in 5–10% of patients with acute cholecystitis.16 It is suggested by pericholecystic fluid and the features of gangrenous cholecystitis. Localised disruption of the gallbladder wall is seen on US in 40% and on CT in 80% (Fig. 32-14). Less often, generalised peritoneal fluid may be present.18 CEUS can help identify perforation by showing local absence of gall-bladder wall enhancement.
Emphysematous Cholecystitis
This condition accounts for only 1% of acute cholecystitis but has a relatively high mortality rate. It is more common in men (the reverse of the usual female predominance in cholecystitis), about 50% are diabetics, and stones are present in <50%.16 The diagnosis may be evident on plain radiographs (Fig. 32-15) and is readily made on CT (Fig. 32-16A), which shows intramural and/or intraluminal gas caused by gas-forming organisms. On US intramural gas appears as focal or diffuse bright echogenic lines. Intraluminal gas, in the non-dependent portion of the gallbladder, causes a curvilinear, brightly echogenic band with shadowing (Fig. 32-16B), which can make recognition of the gallbladder difficult and lead to a false-negative US result. Small foci of intramural gas may cause ring-down artefact and mimic adenomyomatosis.

Acalculous Cholecystitis
Acute acalculous cholecystitis is most often seen in critically ill patients, and the clinical presentation is usually one of sepsis. The US signs are gallbladder distension, gallbladder wall thickening, echogenic contents and, occasionally, sloughed membranes/mucosa and pericholecystic fluid. Positive diagnosis is often difficult as sludge and gallbladder distension may occur without cholecystitis in this group. All investigations—US, CT and biliary scintigraphy—are less accurate than in acute calculous cholecystitis. Biliary scintigraphy is possibly the most accurate technique. Gallbladder aspiration has been used to aid diagnosis but is often unhelpful.19 Localised gallbladder tenderness is a good predictive sign when present but is frequently difficult to assess in this cohort.
Chronic acalculous cholecystitis is a controversial entity as there are no clear clinical, pathological or imaging criteria for its diagnosis. The clinical setting is usually unexplained biliary-type pain, and patients have often previously undergone numerous other negative investigations. US may show gallbladder wall thickening and, by definition, no stones. Cholescintigraphy followed by the IV infusion of cholecystokinin (CCK), or one of its analogues, can be used to assess gallbladder contractibility. An ejection fraction <35% on CCK-cholescintigraphy is generally taken to be an indicator of gallbladder dysfunction and helps select patients who may benefit from cholecystectomy.
Xanthogranulomatous cholecystitis is an unusual form of chronic cholecystitis that may mimic malignancy. It usually presents with the clinical features of cholecystitis or biliary obstruction (a variant of Mirizzi’s syndrome). It is characterised by focal or diffuse gallbladder wall thickening, which can be marked. Stones are present in the majority, and in a small percentage there is associated gallbladder carcinoma.20
Gallbladder Mucocele
Gallbladder mucocele, also known as gallbladder hydrops, is the result of chronic gallbladder obstruction, without superimposed infection, allowing accumulation of large volumes of sterile mucinous fluid. It is usually caused by impacted stones and less often by polyps, tumours or adjacent adenopathy. The gallbladder is markedly distended, fluid filled and may present as a mass.
Gallbladder Fistulae
Gallbladder fistulae are rare. The great majority are due to chronic stone disease rather than neoplasm. Most communicate with the duodenum and most of the remainder to the colon. Cholecystoduodenal fistulae may result in bowel obstruction due to the impaction of larger stones in the distal small bowel, so-called gallstone ileus, a condition associated in a minority of patients with a visible gallstone on plain radiographs or CT, and gas in the biliary tract.
Porcelain Gallbladder
Porcelain gallbladder is an uncommon condition occurring in 0.2% of cholecystectomy specimens21 and consisting of complete or scattered mural calcification. There is an association with gallbladder carcinoma, although the incidence of coexisting carcinoma is less than previously thought at <5%.21 However, prophylactic cholecystectomy is often advocated, due to the high morbidity and mortality of gallbladder carcinoma. The calcification follows the contour of the gallbladder wall, may be focal or diffuse, and may be visible on CT (Fig. 32-17B) or plain radiography. On US (Fig. 32-17A) it can mimic emphysematous cholecystitis or gallstones but the ‘double-arc shadow’ sign of stones is absent.
Adenomyomatous Hyperplasia
This condition is known by several names, including adenomyomatosis and cholecystitis glandularis proliferans. It occurs in up to 9% of cholecystectomy specimens and in 90% of cases there are associated gallstones. It is characterised by thickening of the gallbladder wall due to epithelial and smooth muscle hyperplasia, with cystic epithelial invaginations into the wall (Rokitansky–Aschoff sinuses) and these spaces may contain small stones. Its distribution is fundal (most common), segmental (usually in mid-body) or diffuse. On US it appears as gallbladder wall thickening with secondary luminal narrowing (Fig. 32-18). The affected segment often contains bright echoes arising from the cystic spaces, often associated with ‘comet-tail’ ring-down artefacts. CT shows wall thickening. Contrast gallbladder studies and T2-weighted MR may show the intramural cystic spaces (Fig. 32-19).20 Adenomyomatous hyperplasia is usually asymptomatic, yet it is important to recognise, to avoid misdiagnosis as gallbladder carcinoma. Differentiation of focal forms of adenomyomatosis from small gallbladder carcinomas can be difficult with CT, and ultrasound is usually more reliable.
Gallbladder Polyps
The great majority of polyps are composed of cholesterol and less often are adenomatous. Cholesterol polyps are usually 2–10 mm in size, whereas adenomas can be up to 2 cm. They both appear as small echogenic non-shadowing foci (Fig. 32-6) adherent to the gallbladder wall, often in a non-dependent portion. The main differential diagnosis is small stones and careful US technique can show the subtle, thin, acoustic shadowing diagnostic of a stone. Lack of mobility favours a polyp rather than a stone (Fig. 32-7). True malignant polyps are rare,22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree