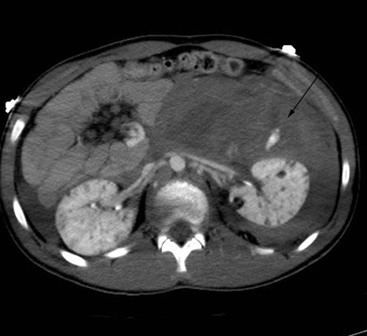
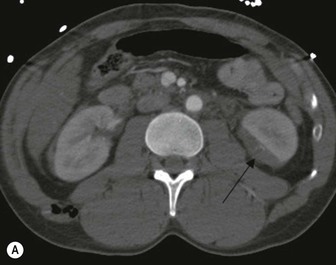
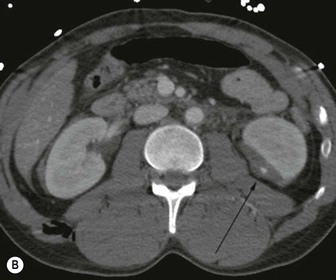
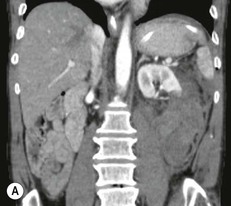
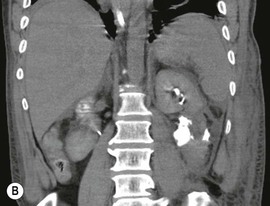
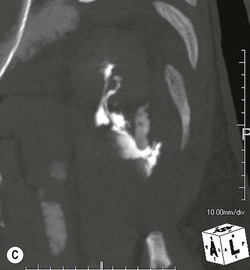
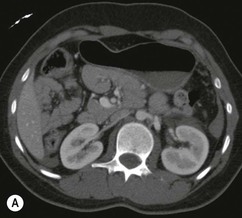
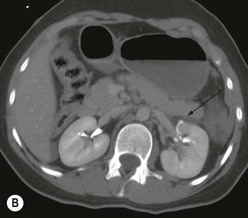
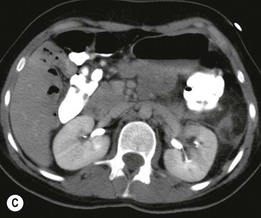
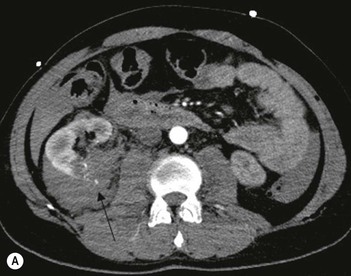
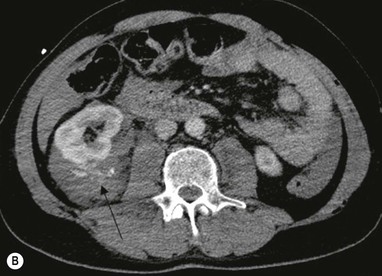
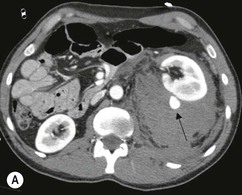
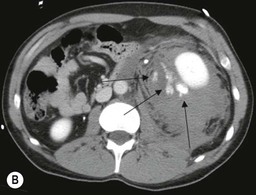
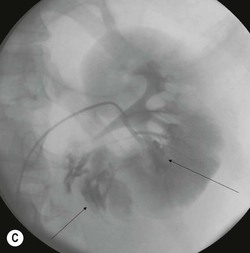
Lisa A. Miller, Stuart E. Mirvis There has been a steady advance in the imaging, management and treatment of genitourinary (GU) system trauma. Multidetector CT (MDCT) has become the primary diagnostic tool for the rapid and accurate assessment of acute injuries of the kidneys, ureters and bladder. Advances in computer speed and use of thinner MDCT slices now allow for rapid reconstruction of axial images and faster generation of 3D multiplanar, maximal intensity projections and volumetric imaging. Use of these non-axial images can enhance visualisation and comprehension of selected urinary system injuries. This chapter describes imaging findings seen with injury to the urinary system and male genitalia from both blunt and penetrating force. The emphasis of the chapter is on the MDCT findings of acute traumatic injury, but also considers complications arising from injuries to these structures. In selected situations, other techniques—including intravenous and retrograde urography, ultrasound and renal nuclear scintigraphy—are required. However, in the acute phase of post-traumatic imaging, MDCT is the most efficient and information-intensive study to assess the injured GU system. Interventional radiology has increasingly been used to manage vascular renal injury and complications of urinary tract injury, such as infected urinoma or partial ureteral tear, thus avoiding the need for surgical exploration. The manner in which diagnostic findings and interventional techniques are integrated with clinical observations and therapeutic decisions will also be described in this chapter. Renal injury is common, occurring in 8–10% of cases of blunt and penetrating trauma.1 About 90% of renal injuries result from blunt force and 10% from penetrating trauma.2 Gunshot or stab wounds are the most common cause of penetrating injury, although iatrogenic injuries sustained during renal biopsy or laparotomy for non-genitourinary disease also unfortunately occur. Patients with an acquired or congenital renal anomaly such as renal transplant, horseshoe kidney, ectopic kidney, renal cyst, renal neoplasm or hydronephrosis are more vulnerable to traumatic renal injury.3 An underlying renal lesion should be suspected if a patient presents with CT findings of renal injury out of proportion to the mechanism of injury (Fig. 43-1).4 The clinical indications for imaging evaluation of the GU system depend on several factors, including the overall haemodynamic status of the patient, other injuries sustained, the site of blunt or penetrating trauma and the presence or absence of gross haematuria. Patients who are haemodynamically unstable and cannot be rapidly resuscitated are usually taken directly to surgery. Although no longer commonly used, a rapid intravenous urogram (IVU) can be performed in the admission area, but is probably best obtained in the operating room, once haemodynamic stability is achieved. This single radiograph will allow visualisation of both kidneys, if functional, and detect the majority of major renal parenchymal injuries.5 IVU is approximately 68% accurate for staging penetrating trauma;6 it may not detect minor renal parenchymal lacerations and contusions, but these are unlikely to have major clinical ramifications. Although ultrasound is often used in the diagnosis of medical renal disease and for rapid assessment of the peritoneal cavity for free fluid, it cannot assess renal function and is relatively insensitive for detection of renal lacerations and contusions, extravasation of blood or urine, collecting system disruption and parenchymal haematoma. Because of these limitations, a negative ultrasound examination cannot exclude a renal injury and is not commonly used in the assessment of acute renal injury.7 The significance of haematuria as an indicator of significant renal injury has been the subject of debate, but a consensus appears to be developing. Hardeman et al. found that 21 of 25 patients (84%) presenting with gross haematuria following blunt trauma had documented renal injury.8 As a rule, all patients with penetrating flank and back trauma should have a CT examination. Microscopic haematuria without hypotension is very unlikely to be associated with major renal injury. Miller and McAninch reviewed 1588 blunt trauma patients without haematuria or shock on admission.9 Only three had significant renal injuries, all of which were diagnosed at laparotomy performed for other indications. A combination of four series comprising 2873 blunt trauma patients with microscopic haematuria, but without shock on admission, reported only 10 with significant renal injuries, most of which were also detected at laparotomy performed for other indications. These authors also reported that 78 of 422 patients admitted with gross haematuria or microscopic haematuria with shock had grade II–V renal injuries, of whom 34 required repair. Haematuria may be absent in patients with severe renal injury, particularly those with vascular pedicle injury, ureteral tear, ureteropelvic disruption and penetrating urinary system injury.10 One series reported a normal urinalysis in 24% of patients with major renal artery occlusion.8 However, in the paediatric population, Stein et al. have shown that microscopic haematuria without hypotension can be associated with significant renal injury, and recommend CT imaging for both gross and microscopic haematuria.11 Several other clinical factors should also be considered and are reflected in the imaging guidelines utilised at the Maryland Shock Trauma Center, as listed in Table 43-1. TABLE 43-1 Indications for Renal Imaging in Acute Trauma In general, the kidneys and proximal collecting systems are evaluated as part of the abdominal-pelvic CT study without special protocols. In our centre, the abdomen and pelvis are examined using 40- or 64-channel MDCT with non-ionic IV contrast material as part of a whole-body trauma CT study. An intravenous bolus of 100 mL iodinated contrast agent (350 mg/mL) is given at 6 mL/s for 60 mL and then 4 mL/s for 40 mL by power injector. CT data acquisition is triggered to begin when a threshold density of 100 HU is reached in the ascending aorta. Axial images at 3–5 mm are fused for review and storage on a PACS workstation. The original images are used for all 2D or 3D reformatted imaging and are also saved to a proprietary reconstruction workstation for reference, if required. Since MDCT covers the abdomen and pelvis very quickly, there is no opportunity for opacification of the renal pelvis and ureters, resulting in failure to diagnose potential proximal collecting system injury.12 Currently, at our centre, if there is radiological suspicion for collecting system injury on the initial CT image set, additional 3- to 20-min delayed images may be obtained as necessary to evaluate for extravasation of intravenous contrast material from the collecting system or ureters. Reduced radiation dose is adequate for additional delayed images since these images are used primarily for detection of high-density contrast material rather than parenchymal injury.13 Delayed CT has been shown to detect as many as 8.6% of collecting system injuries in patients with blunt renal trauma.12 The arterial phase images are most useful in demonstrating presence and symmetry of IV contrast by the kidneys and potential active bleeding or traumatic pseudoaneurysm, while the portal venous images provide more information about the extent of parenchymal damage and help differentiate active bleeding from traumatic pseudoaneurysm. Management of renal trauma depends to a large degree on extent of the injury and clinical status of the patient. In 1989, the American Association for the Surgery of Trauma (AAST) created a renal organ injury grading system, which was based on surgical observations. This grading system was found to be valid in that increasing renal injury grade was directly correlated with the need for renorrhaphy or nephrectomy,14 the need for haemodialysis and inpatient mortality after blunt renal injury, the need for nephrectomy after penetrating trauma15,16 and decreased renal function after major renal injury.17 In 2011, a review of 3580 renal injuries by Buckley and McAninch18 led to the revision of the original AAST renal grading system (Table 43-2). The 2011 revision is not solely based on surgical findings but takes into account radiological findings. The revised grading system includes renal injuries not described in the original grading scale (segmental vascular injuries, ureteral pelvic injuries) and reclassifies grade IV and grade V injuries. In the revised grading system, grades I–III remain the same as the original 1989 renal injury staging. Surgical literature was inconsistent in the definition of a grade IV or V injury and no clear management and outcome data could be obtained for these severe injuries. Grades IV and V were thus reclassified in the revised system with more precise language to optimise management, standardise clinical research and improve outcomes. TABLE 43-2 Revised AAST Renal Injury Grading System A renal unit can sustain more than one grade of injury and should be classified by the higher grade of renal injury. Generally, patients with grades I–III renal injuries are managed non-operatively. With the gradual shift in trauma care to a more conservative approach, most revised AAST grade IV injuries will likely be managed with interventional angiography and active surveillance, with the exception of a complete ureteral pelvic junction avulsion, which requires surgery, while most grade V injuries will likely have a higher exploration rate and lower renal salvage rate. Our institution uses a CT-based grading scale similar to the revised AAST renal injury grading system (Table 43-3). Most renal injuries are minor (75–98%), represented by grades I–III, and will heal spontaneously without intervention. Contusions are visualised as ill-defined low-attenuation areas with irregular margins. They may appear as regions with a striated nephrogram pattern due to differential blood flow through the contused parenchyma. These lesions do not usually require follow-up imaging. Subcapsular renal haematomas are uncommon as the renal capsule is not easily separated from the cortex. A subcapsular haematoma appears as an unenhanced, typically convex fluid collection indenting the underlying parenchyma (Fig. 43-2). Small subcapsular haematomas may become biconvex as they enlarge (Fig. 43-3). Some delay in renal perfusion may be seen with these injuries, secondary to increased resistance to arterial perfusion. In most cases the injury will resolve without specific treatment, although acute or delayed onset of hypertension from renal parenchymal compression (Page kidney) should be sought. Large subcapsular haematomas could theoretically compress the kidney to near systolic level pressures, preventing perfusion and requiring surgical release of renal tamponade. Minor renal lacerations can be either superficial, involving the cortex only, or deep, extending to the renal medulla. Lacerations appear as non-enhancing linear defects on a background of otherwise normally enhancing renal parenchyma (Fig. 43-4). As a rule, a minor renal laceration will spare the collecting system. These are usually self-limited injuries, typically accompanied by small amounts of perinephric haemorrhage. Perinephric haemorrhage will appear as a poorly marginated, hyperdense fluid confined by Gerota’s fascia. Thickening of the lateral conal fascia may also be seen (Fig. 43-5). Even if the perinephric haematoma is large, it will usually not indent the renal contour as seen with a subcapsular haematoma. Segmental renal infarctions are also considered minor renal injuries and are relatively common in blunt renal trauma. These result from stretching and subsequent occlusion of an accessory renal artery, extrarenal or intrarenal branches of the renal artery or a capsular artery.19 Segmental infarctions appear as sharply demarcated, wedge-shaped areas of very low attenuation with the base of the wedge at the renal capsule and the apex at the renal hilum. Segmental infarctions typically involve the renal pole(s). More significant renal trauma may require intervention by angiography or surgery. AAST grade IV injuries include deep renal lacerations that extend into the renal collecting system with or without urinary extravasation, segmental vascular injury within the kidney and renal pelvis laceration or ureteropelvic junction disruption. Lacerations of the collecting system are identified on CT by extravasation of urine into the perirenal space (Fig. 43-6). A collecting system injury can be missed if the CT images are obtained before intravenous contrast has reached the collecting system. Visualisation of any fluid around the kidney on the admission CT may require additional, delayed CT imaging when appropriate. Delayed images will show the collection of high-density, contrast-enhanced urine adjacent to the site of injury. If the injury is not diagnosed on CT, the development of sepsis, decreasing renal function or unexplained increasing serum creatinine and blood urea nitrogen (BUN) should raise concern for urine leak. Urine leaks from collecting system injury spontaneously resolve in 87% of cases and can often be managed with observation alone (Fig. 43-7). Resolution is particularly likely if there is unimpeded antegrade urine flow. Urinomas can become infected due to urine stasis or bacterial contamination from penetrating injury, and are managed by percutaneous drainage. Persistent collecting system leaks may be treated with nephrostomy or a double-J ureteral catheter. Surgical repair may be required if non-surgical interventions do not resolve the leak. This is more likely in cases where the urine leak communicates with a low-pressure space like the pleural or peritoneal cavity. Segmental vascular injuries include active bleeding and traumatic pseudoaneurysm formation. Active bleeding into the kidney or surrounding tissue appears as patchy or linear dense contrast material surrounded by less dense haematoma on arterial phase images. On portal venous imaging, the area of extravasated contrast material will increase (Fig. 43-8) and remain high in density. Haemorrhage is apparent because arterial extravasation appears before opacification of the renal collecting system. Typically, the density of extravasated arterial contrast medium is greater than 80 HU and is within 15 HU of an adjacent artery (Fig. 43-9).20 A traumatic renal pseudoaneurysm will appear as a rounded or oval area of high density on arterial phase imaging that becomes isodense on portal venous images (Fig. 43-10). In stable patients, sites of active bleeding and traumatic pseudoaneurysms can be confirmed by selective renal angiography and treated by angioembolisation with Gelfoam pledgets followed by coils.
Genitourinary Tract Trauma
Introduction
Renal Injury
Clinical Aspects
Indication
Imaging Study
Penetrating flank and back trauma
Chest, abdominal-pelvic CT with IV and oral contrast medium
Gross haematuria
Abdominal-pelvic CT with oral and IV contrast medium if haemodynamically stable or resuscitated
Haemodynamically unstable requiring emergency surgery
Intraoperative IVU when stabilised
Haemodynamically stable with microscopic haematuria, but no other indication for abdominal-pelvic CT
Observation until resolution of haematuria
Haemodynamically stable with microscopic haematuria, but other indications for abdominal-pelvic CT (+ abdominal examination, decreasing haematocrit, indeterminate result of peritoneal lavage or abdominal ultrasound, unreliable physical examination)
Abdominal-pelvic CT with oral and IV contrast medium
Haemodynamically stable with or without microscopic haematuria with evidence of major flank impact (e.g. lower posterior rib or lumbar transverse process fracture, major contusion of flank soft tissues)
Abdominal-pelvic CT with oral and IV contrast medium
CT Technique for Renal Injury
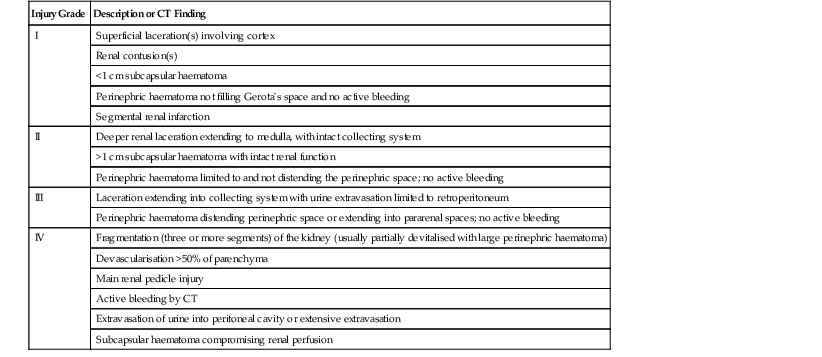
Grading of Renal Injury and Implications for Management
Grade
Injury Location
Definition
I
Parenchyma
Subcapsular haematoma and/or contusion
Collecting system
No injury
II
Parenchyma
Laceration <1 cm in depth and into cortex, small haematoma contained within Gerota’s fascia
Collecting system
No injury
III
Parenchyma
Laceration >1 cm in depth and into medulla, haematoma contained within Gerota’s fascia
Collecting system
No injury
IV
Parenchyma
Laceration through the parenchyma into the urinary collecting system
Vascular segmental vein or artery injury
Collecting system
Laceration, one or more into the collecting system with urinary extravasation
Renal pelvis laceration and/or complete ureteral pelvic disruption
V
Vascular
Main renal artery or vein laceration, avulsion or thrombosis
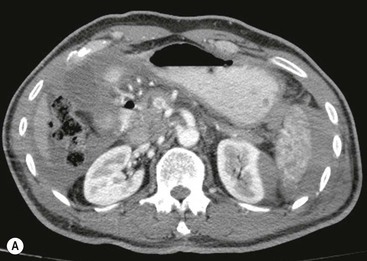
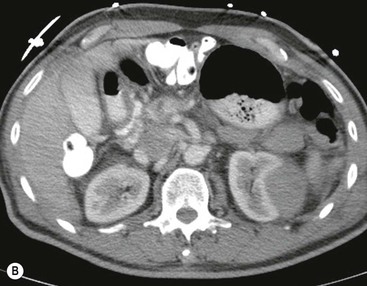
CT of Grade I–III Renal Injury
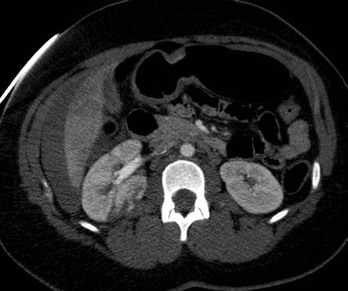
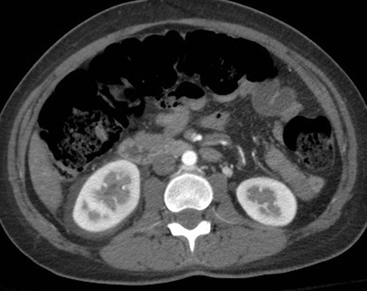
CT of Grade IV Renal Injury