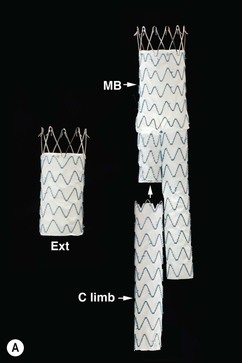
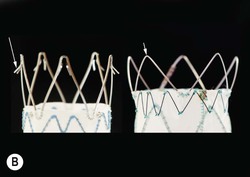
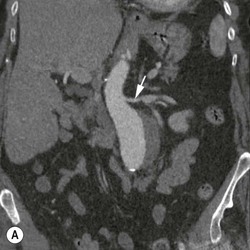
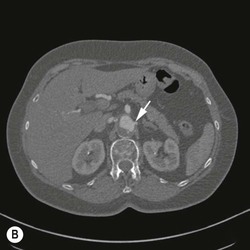
Christopher J. Hammond, Anthony A. Nicholson Open surgical treatment of aortic pathology is often technically demanding for the surgeon and invasive for the patient, being associated with a significant physiological insult. Surgery for the thoracic aorta requires thoracotomy or median sternotomy, aortic cross-clamping, often single lung ventilation and sometimes cardiopulmonary bypass or circulatory arrest. Surgery for the abdominal aorta requires laparotomy, or extensive retroperitoneal dissection, medial visceral rotation and aortic cross-clamping. The aortic tissue is usually diseased and may be friable (especially in the case of dissection and intramural haematoma (IMH)) or calcified and brittle. In either situation, working with, dissecting and suturing the aorta is challenging. The cohort of patients requiring aortic intervention is usually that least able to withstand it. In most cases, aortic pathology is associated with generalised cardiovascular disease, including ischaemic heart disease, cerebrovascular, renovascular and peripheral vascular disease as well as other comorbidites such as respiratory impairment. Mortality rates for elective open surgery for thoracic and abdominal aneurysms are in the region of 8 and 5%, respectively,1,2 and morbidity is significant. These considerations have driven the development of minimally invasive endovascular therapies for aortic disease. Endovascular repair of the aorta using covered stents (stent-grafts) was first described in 19913 and is generally associated with a smaller physiological insult and lower mortality than open surgical repair.2,4 However, there are a number of unique limitations (particularly relating to anatomy) and complications. Although the current generation of stent-grafts is easier to insert and performs better in the long term than their predecessors, they are by no means a panacea for all aortic pathology. A multidisciplinary approach to aortic disease is vital and should include cardiothoracic and vascular surgeons, interventional radiologists, vascular anaesthetists and device specialists (e.g. company representatives). This multidisciplinary approach should ensure that the patient receives the most appropriate treatment, be it open surgery, endovascular repair or medical management. This chapter will discuss the endovascular management of aortic pathology and in particular the indications, anatomical and technical considerations and complications of the technique. A stent-graft (Fig. 85-1) is composed of a fabric tube, usually woven polyester or expanded polytetrafluoroethylene (Gore-Tex). Circular or crown-shaped metal struts (‘ring stents’), usually made of nitinol or Elgiloy, are attached to the tube by either suturing or gluing. The exact design of the ring stent varies from manufacturer to manufacturer, but in all cases serves to provide support for the fabric and provide firm apposition of the stent-graft to the wall of the aorta. Some devices have uncovered stents at one end to provide additional support and anchorage above or below the covered portion of the device. The stent-graft is held in place by the radial force of the ring stents, which produce friction against the aortic wall and prevent distal device migration. Additionally, some devices have small hooks (usually at the top end of the fabric or on an uncovered stent) which engage with the aortic wall to prevent migration (Fig. 85-1B). Most manufacturers make a range of stent-graft diameters and lengths to allow ‘off the shelf’ device selection. Complex devices (such as fenestrated or branched devices or devices in sizes outside the normally manufactured ranges) can be ordered but are expensive and there is a lead-time associated with their manufacture. Currently (July 2013), the approximate cost of a typical single thoracic aortic stent-graft (Fig. 85-1C) is £10k. A bifurcated abdominal aortic device costs about £6k and a custom-made device can cost £15k or more. Good quality high-resolution imaging is required to choose the correct stent-graft for a particular aneurysm. This is usually achieved with thin-section contrast-enhanced arterial phase computed tomography (CT) to include the whole of the diseased section of the aorta and the access vessels (see below) in a volumetric acquisition. Magnetic resonance (MR) angiography is sometimes utilised. Multiplanar reformatting software is essential to allow measurement of true axial diameters and vessel length. Expert systems are available to automate this process, though many clinicians prefer to do it by hand. To achieve a good ‘seal’ between the device and the aortic wall, the stent-graft is usually oversized relative to the aorta by 10–20%. The aorta at the point of seal (sometimes called the ‘neck’) should be relatively disease free and straight-sided. Most stent-grafts require a neck length of 8–15 mm to achieve a seal. Shorter necks (Fig. 85-2A) and diseased necks (e.g. those containing marked atheroma or thrombus, Fig. 85-2B) risk a suboptimal seal and leakage between the device and the aortic wall. A neck which is sharply angulated relative to the more distal aorta (Fig. 85-2C) risks the stent-graft not deploying truly perpendicular to the aortic wall. This can increase the risk of suboptimal apposition and leak, though modern devices are designed to accept a greater degree of neck angulation than earlier designs, and some devices are specifically marketed for greater neck angulation. This requires specific design features to prevent graft lumen collapse. Finally, markedly barrel or conical shaped necks (Fig. 85-2D) increase the risk of poor seal as the degree of oversize needed to achieve a seal at the narrower portion of the neck may not be sufficient to achieve seal at the wider portion. The considerations outlined above for the ‘neck’ apply equally to the distal sealing point, often called the ‘landing zone’. Approximately 40% of abdominal aortic aneurysms are unsuitable for conventional (as opposed to fenestrated or branched) endovascular repair because of adverse morphology.5 A description of the degree to which an operator can compromise on an ‘ideal’ neck (minimal angulation, straight-sided, long and disease free) is beyond the scope of this chapter though there are many reports of operators deploying devices in ‘off-label’ morphologies (where the characteristics of the aorta are outwith the published ‘indications for use’ (IFU) limits of the device) with satisfactory results.6,7 Stent-grafts are supplied preloaded on a deployment system. They are usually constrained within a sheath, which, at deployment, is gradually withdrawn, allowing the stent to expand under its own radial force. Many delivery systems have mechanisms to allow partial deployment and repositioning before final release. The ‘windsock’ effect of systolic pressure during the cardiac cycle forcing a partially deployed device distally from its intended deployment position is occasionally problematic, though usually only in the proximal thoracic aorta. Some thoracic delivery systems have mechanisms to constrain the proximal portion of the stent-graft until the remainder of the device is fully deployed to minimise this windsock effect. Where the proximal positioning of a thoracic stent-graft is critical (e.g. in short or angulated necks) overdrive cardiac pacing or pharmacological manipulation to lower blood pressure may be used as the device is deployed. Once completely released, a device cannot be retrieved or repositioned without open surgery. Deployment systems vary in diameter between 14 and 25Fr (approximately 4–9 mm). Patients in whom the access vessels (usually the common femoral and iliac arteries) are narrower than the diameter of the delivery system required (Fig. 85-2E) cannot undergo endovascular repair without adjunctive procedures such as previous angioplasty or stenting of the stenosed segment or the placement of a temporary surgical conduit to allow the device to be inserted from above any stenosis. Heavily calcified or tortuous access vessels may also preclude endovascular repair as they will not straighten sufficiently to allow passage of the delivery system. Attempts to force a large delivery system through suboptimal access can result in access vessel rupture or avulsion with potentially disastrous consequences. Table 85-1 summarises the absolute and relative contraindications to endovascular repair of aortic pathology with conventional stent-grafts. The characteristics of some currently manufactured stent-grafts are summarised in Table 85-2. TABLE 85-1 Absolute and Relative Contraindications to Endovascular Repair of Aortic Pathology TABLE 85-2 Summary of Characteristics of Some Currently Manufactured Aortic Stent-Grafts * IFU = Indications for use. ** Diameter (mm) = French size /π. Endovascular repair of aortic pathology is associated with a number of novel complications that may occur months or years following the procedure. For this reason, patients undergoing endovascular repair undergo a programme of surveillance imaging over a number of years (and sometimes indefinitely) following the procedure. The exact details of the surveillance vary from institution to institution though most will involve cross-sectional imaging initially (arterial phase CT or MR angiography at 1 or 3 months, or both) and regularly (at least annually) thereafter for a number of years. Plain radiographs of the device are also obtained to assess for ring stent fracture, device dislocation and migration. Ultrasound (US) and contrast-enhanced US may be used to detect sac expansion and endoleak. Some authors have advocated replacing CT with US as early as 1 year post-abdominal EVAR (within certain strict criteria) to minimise radiation, dose of iodinated contrast agent, cost and inconvenience to the patient.8 Such programmes of less intensive surveillance will evolve as device performance improves with successive stent-graft generations. The novel complications of endovascular repair are endoleak, device migration and dislocation, kinking and occlusion (Fig. 85-3). This is defined as continuing flow of blood into the diseased segment of aorta outside the lumen of the stent-graft. There are five types of endoleak described. Poor sealing between the device and the aortic wall, at both the proximal and distal seals (the neck and distal ‘landing zone’), can result in leakage of blood between the aortic wall and the device and incomplete exclusion of the treated segment of the aorta from the circulation. This is a type 1 endoleak. It is often associated with adverse neck morphology9,10 and sometimes with device migration over time. Errors in device sizing can also be a cause. Type 1 endoleak is associated with poor long-term outcome, with ongoing sac pressurisation, expansion and rupture.11 It typically requires treatment. Therapeutic options include insertion of proximal or distal extension cuffs, balloon moulding or restenting of the seal zones, open surgical buttressing, device explantation and repair or attempts at transcatheter embolisation of the endoleak. Small side branches of the aorta (e.g. the intercostal, lumbar or inferior mesenteric arteries) are not usually occluded during endovascular repair. This allows the possibility of retrograde flow of blood into the diseased segment of aorta via these side branches—a type 2 endoleak. These endoleaks often cease spontaneously. They do not require treatment in the absence of aneurysm sac or false lumen expansion.11 Such expansion is uncommon, but, if it occurs, it can cause alteration in the morphology of the proximal or distal stent-graft seal zones with eventual loss of seal and type 1 endoleak (which would require treatment). Therefore type 2 endoleaks with ongoing expansion are treated, usually with embolisation. Routine embolisation of prominent side branches before stent-graft deployment is time-consuming and has not been shown to alter rates of subsequent sac expansion.12 The exceptions to this are the left subclavian artery and the internal iliac artery, both of which are moderately large vessels with prominent collateral circulation and, untreated, are a source of significant type 2 endoleak. Graft defects and fabric tears are rare with modern devices but for modular devices (such as bifurcated abdominal aortic stent-grafts, or long segment thoracic aortic devices) the seal between components can be incomplete or dislocation of one component from another can occur. The associated type 3 endoleak causes repressurisation of the diseased segment of aorta and (for aneurysmal disease) sac expansion and rupture. It requires treatment, usually by balloon moulding any joints, relining defects with a secondary device or operative repair. Type 4 endoleak refers to transient graft ‘porosity’—equivalent to the ‘sweating’ sometimes seen with knitted open surgical grafts. It is rare with modern stent-graft fabric and does not require treatment. Type 5 endoleak refers to ongoing aneurysm sac expansion in the absence of any other demonstrable endoleak. It may be due to unidentified very low volume or intermittent endoleak, osmotic effects of dissolving atheroma and thrombus or low-grade infection. Treatment, if required (e.g. if the proximal or distal seal is threatened), can be difficult and complex. Each cardiac cycle produces a force estimated to be approximately 10 N (equivalent to 1 kg) tending to push the graft distally in the aorta. This can result in graft migration over time, especially where the proximal seal zone is short or diseased when device apposition to the wall of the aorta is suboptimal. Device migration can result in limb kinking (Fig. 85-3E), which predisposes to occlusion or thrombus formation and distal embolisation. Migration can also result in type 1 or 3 endoleak (if the device migrates out of the neck, or the migration results in a change in device geometry and dislocation of modular components—Fig. 85-3F). Longer-term morphological changes in the treated aortic segment can alter device geometry and cause kinking and dislocation in the absence of migration. Device kinking and migration are rare with modern devices, occurring in approximately 1 and 2% of patients, respectively.5 If there is little risk of complication associated with the migration or kinking (e.g. a small amount of migration in a long neck or a minor kink unlikely to cause haemodynamically significant stenosis), no treatment is required. However, if needed, treatment can be complex. Options include extension stent-grafting, device reinforcement with stent-grafts or uncovered stents, surgical buttressing of the neck or device explantation and open repair. The management of patients with aortic disease is complex and should not be undertaken without adequate supportive infrastructure, staffing, processes and governance. The decision on how (and whether) to treat relies on detailed anatomical, physiological and clinical information. Electively, patients should undergo a formal assessment of their general cardiovascular fitness (such as a cardiopulmonary exercise test (CPX)) with treatment and optimisation, where possible, of any underlying pathology. Once clinical information and data on aortic morphology and cardiovascular fitness are available, the patient should be discussed at a multidisciplinary team meeting and a plan for treatment constructed. Written information should be provided to the patient about their aortic pathology and the proposed treatment. In an emergency such detailed work-up is clearly impossible, though there is occasionally time to optimise the patient’s physiology to an extent. Endovascular aortic repair should be carried out by experienced staff in a sterile environment of theatre standard with optimal imaging facilities and equipment to convert rapidly to open repair in an emergency. The ideal is an angiography theatre with fixed C-arm image intensification and theatre-grade air change and lighting, anaesthetic and surgical equipment, piped gases and suction and facilities for rapid (level 1) infusion and cell salvage. Postoperative care in an intensive-care or high-dependency unit should be available if needed with facilities for invasive ventilation and renal support. Vascular surgical, anaesthetic and radiological support should be available on a 24/7 basis. Written protocols for accelerated postoperative recovery, mobilisation and discharge are desirable. Data should be submitted to national or international registries (such as the National Vascular Database (NVD) in the UK). There is evidence of improved outcomes for open abdominal aortic aneurysm (AAA) repair at centres that undertake a greater volume of work.14 This is almost certainly as much to do with pre- and post-procedural process as it is to do with individual surgical competence. There is likely to be a similar relationship for endovascular repair of all aortic lesions.15 Centralisation of services for aortic repair is being undertaken currently in the UK. For the purposes of endovascular repair, thoracic aortic pathology is best classified according to its location relative to the left subclavian artery (the Stanford classification, described originally for thoracic aortic dissection— see Fig. 85-4). Pathology affecting the aortic root and ascending aorta (Stanford A disease) is generally unsuitable for endovascular repair due to the frequent involvement of the aortic valve (which may also need repair) and close association of critical branch vessels (the coronary arteries and great vessels). There are occasional situations where endovascular intervention is preferable, though at present these must be considered experimental.16 Disease affecting the aorta distal to the left subclavian artery (Stanford B disease) is amenable to endovascular repair, assuming there is enough disease-free aorta proximally to achieve a seal (usually 15–20 mm). The left subclavian artery can be covered by the device to increase the available useable neck proximal to the diseased aorta without significant morbidity17
Aortic Intervention
Introduction
Stent-Grafts and Basic Principles of Stent-Grafting
Contraindication
Comment
Absolute
Patient preference for open surgical repair
Limited life expectancy
Where the risks of intervention outweigh the benefits
Poor cardiopulmonary reserve or other comorbid condition
Where the risks of intervention outweigh the benefits
Significant (>50%) or circumferential thrombus at seal zone
Unstable patients
Where they are deemed unable to withstand the small delay associated with imaging of the pathology
Ascending aortic pathology
Unless there is absolutely no surgical alternative
Relative
Contrast allergy
Procedures can be performed with carbon dioxide angiography or intravascular ultrasound as imaging guidance
Renal insufficiency
Procedures can be performed with carbon dioxide angiography or intravascular ultrasound as imaging guidance
Suboptimal proximal seal zone (‘neck’)
Proceed accepting an increased risk of endoleak, or use fenestrated or branched device
• Angulation
• Length
• Shape
Poor access vessels
Can be mitigated with angioplasty or proximal surgical conduit
• Stenosis
• Tortuosity
• Calcification
Children
Where physiological growth may result in loss of seal and device dislocation
Young patients
As long-term durability of endovascular repair has not yet been established
Not Contraindications
Rupture
Malignancy
Mycotic aneurysms
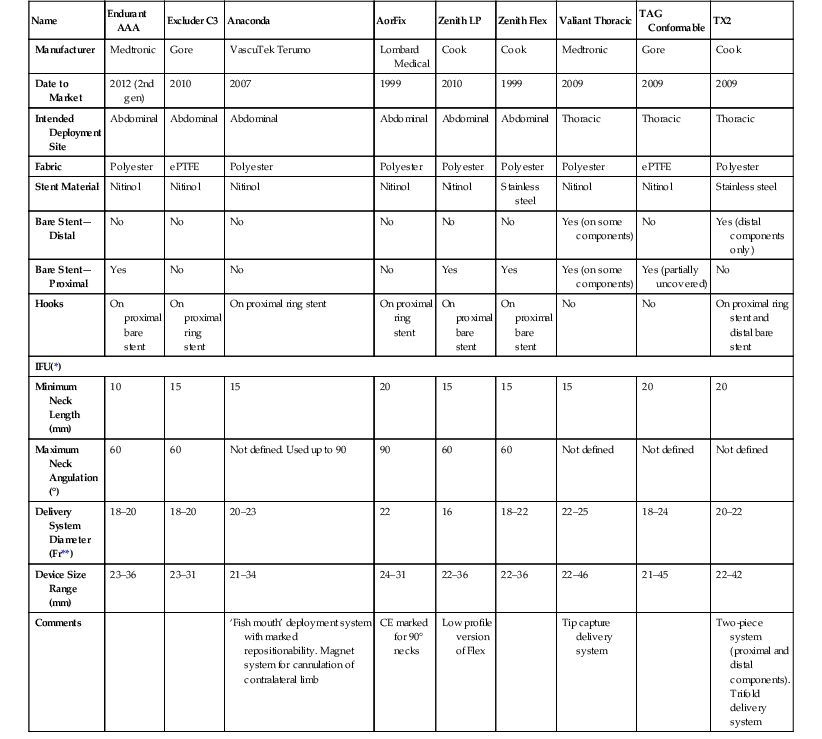
Name
Endurant AAA
Excluder C3
Anaconda
AorFix
Zenith LP
Zenith Flex
Valiant Thoracic
TAG Conformable
TX2
Manufacturer
Medtronic
Gore
VascuTek Terumo
Lombard Medical
Cook
Cook
Medtronic
Gore
Cook
Date to Market
2012 (2nd gen)
2010
2007
1999
2010
1999
2009
2009
2009
Intended Deployment Site
Abdominal
Abdominal
Abdominal
Abdominal
Abdominal
Abdominal
Thoracic
Thoracic
Thoracic
Fabric
Polyester
ePTFE
Polyester
Polyester
Polyester
Polyester
Polyester
ePTFE
Polyester
Stent Material
Nitinol
Nitinol
Nitinol
Nitinol
Nitinol
Stainless steel
Nitinol
Nitinol
Stainless steel
Bare Stent—Distal
No
No
No
No
No
No
Yes (on some components)
No
Yes (distal components only)
Bare Stent—Proximal
Yes
No
No
No
Yes
Yes
Yes (on some components)
Yes (partially uncovered)
No
Hooks
On proximal bare stent
On proximal ring stent
On proximal ring stent
On proximal ring stent
On proximal bare stent
On proximal bare stent
No
No
On proximal ring stent and distal bare stent
IFU(*)
Minimum Neck Length (mm)
10
15
15
20
15
15
15
20
20
Maximum Neck Angulation (°)
60
60
Not defined. Used up to 90
90
60
60
Not defined
Not defined
Not defined
Delivery System Diameter (Fr**)
18–20
18–20
20–23
22
16
18–22
22–25
18–24
20–22
Device Size Range (mm)
23–36
23–31
21–34
24–31
22–36
22–36
22–46
21–45
22–42
Comments
‘Fish mouth’ deployment system with marked repositionability. Magnet system for cannulation of contralateral limb
CE marked for 90° necks
Low profile version of Flex
Tip capture delivery system
Two-piece system (proximal and distal components). Trifold delivery system
Surveillance Imaging and Complications
Endoleak
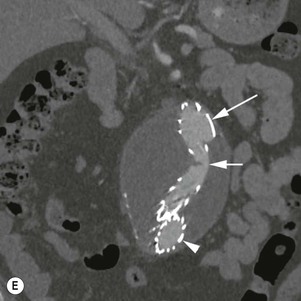
Type 1 Endoleak (Fig. 83-3A)
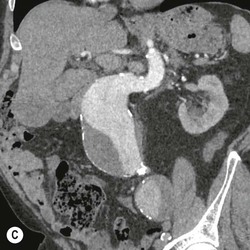
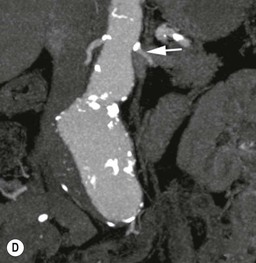
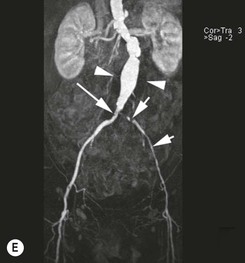
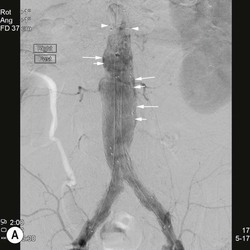
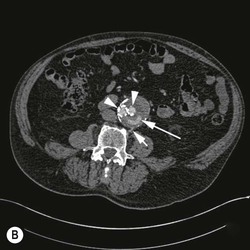
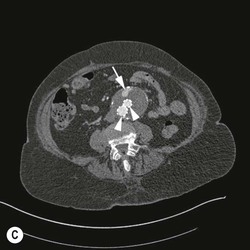
Type 2 Endoleak (Figs. 85-3B and C)
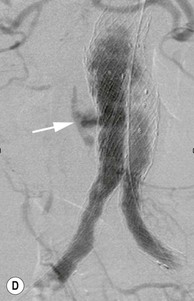
Type 3 Endoleak (Fig. 85-3D)
Types 4 and 5 Endoleak
Device Migration, Dislocation, Kinking and Occlusion
Infrastructure and Staffing13
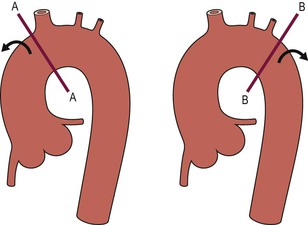
Thoracic Aortic Intervention
Anatomical Considerations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aortic Intervention
Chapter 85