Figure 1.1 Proximal femur from an older child demonstrating the cortex, medullary cavity, growth plates, apophysis, and articular surface.
Tan-white and smooth-surfaced, bones are the hardest and strongest structures of the body, being as strong as cast iron but one-third of the weight as a result of their adaptive architecture. Comparatively lightweight, rigid but not brittle, reinforced, generally asymmetric, and hollow, bones are designed to have a relatively high tensile strength, and maximum strength-to-weight ratio. These characteristics are derived from the substance of bones – all are composed of bone tissue – a specialized type of connective tissue that is a unique biphasic blend of inorganic or mineral component – calcium hydroxyapatite – and organic constituents – the cells and the proteins they synthesize.
Bones vary greatly in size and shape and these features form the basis of the classification of individual bones. The most prominent group of bones are tubular, both long and short (Fig. 1.1), and the other types include flat (bilaminar plates) and cuboid bones. Anatomically, tubular bones are further subdivided into the epiphysis, the metaphysis, and the diaphysis (Fig. 1.2) (2). The epiphysis extends from the base of the articular surface to the beginning point of significant narrowing of the bone. The metaphysis embodies the region of bone that displays a prominent reduction in diameter, and the diaphysis or shaft extends from the base of one metaphysis (the point where decrease in bone diameter ceases) to the base of the opposing metaphysis. During growth and development the metaphysis is composed of the cartilaginous growth plate also known as the physis and the adjacent primary and secondary spongiosa and the surrounding cortex. The medical and forensic determination of skeletal age and the prediction of ultimate size utilize the degree of maturation of the physes, the amount and localization of bone ossification, the formation and dimensions of the secondary ossification centers, and the degree and amount of remodeling (see below).
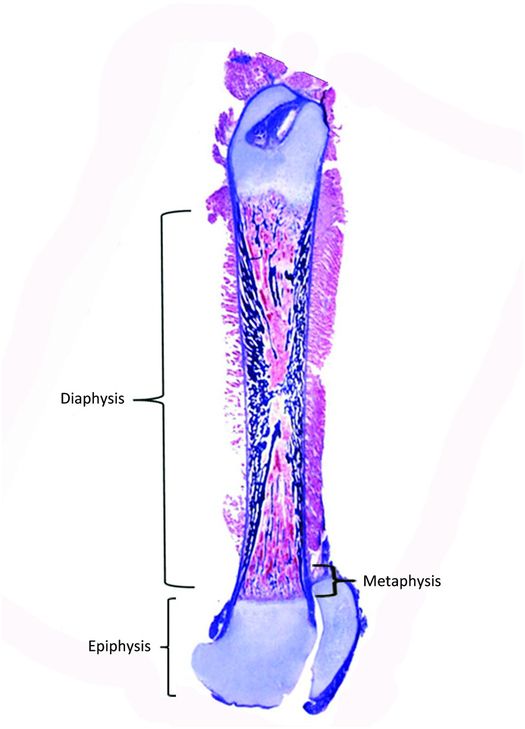
Figure 1.2 Femur from a fetus showing the epiphysis, metaphysis, and diaphysis.
Bones are covered externally by a periosteum. The periosteum is anchored to the cortex, which in turn houses the medullary canal that contains variable amounts of cancellous or trabecular bone, fatty and hematopoietic marrow, blood vessels, and nerves. The quantity and arrangement of cortical and cancellous bone is directly related to the biomechanical requirements of each bone. For example, long bones that are exposed to the largest torsional and load-bearing forces and flat bones that serve a protective function, such as the skull, are composed roughly of 80–100% cortical bone and 0–20% cancellous bone. In contrast, bones that transmit predominately weight-bearing forces, such as the vertebral bodies, consist of 80% cancellous bone and 20% cortical bone. The trabeculae of cancellous bone are arranged according to the lines of stress to which they are exposed.
Bone structure
Woven and lamellar bone
Bone tissue is categorized into woven and lamellar types based on the organization of its main structural protein – type I collagen fibers. In woven bone, the collagen fibers are arranged in a seemingly haphazard feltwork, while in lamellar bone they are deposited in parallel arrays (Fig. 1.3).
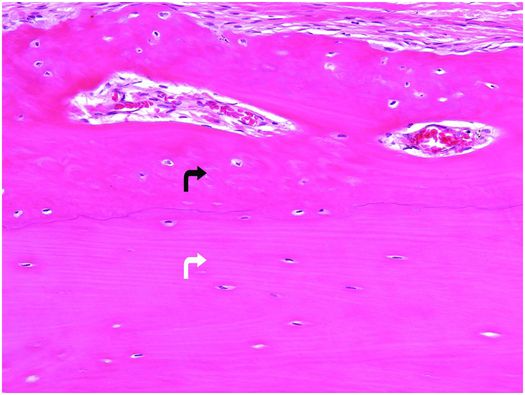
Figure 1.3 Outer portion of cortex composed of lamellar bone (white arrow) that has superiosteal reactive woven bone (black arrow) on its surface. The type I collagen fibers in the woven bone are oriented in a weave, whereas those in the underlying cortex are arranged in parallel array. The osteocytes in the woven bone are more numerous and larger than those in the lamellar bone.
Woven bone is fabricated during periods of rapid bone growth or formation. It composes parts of the developing skeleton during embryogenesis and portions of bones in the growing infant and adolescent. It may also be the predominant type of bone that is formed in a variety of reactive (fracture-callus, infection-involucrum) and neoplastic (Codman’s triangle, matrix of bone-forming neoplasms) conditions. In the adult, woven bone is always pathologic, except at tendoligamentous insertion sites, where small amounts of woven bone are often present.
Histologically, woven bone is hypercellular, and the osteocytes and their lacunae are large and appear to be distributed in a haphazard fashion as the long axes of the cells parallel the differing orientation of the neighboring collagen fibers (Fig. 1.3). Overall, this structural organization enables woven bone to resist forces equally in all directions and facilitates rapid formation, mineralization, and resorption. These factors explain why woven bone is weaker, less rigid, and more flexible than lamellar bone.
Normally, the entire mature skeleton is composed solely of lamellar bone. In contrast to woven bone, lamellar bone is synthesized more slowly, is less cellular, and the osteocytes and their lacunae are smaller and distributed amongst the regularly oriented collagen lamellae, giving it a more orderly appearance (Fig. 1.3). Since the mineral and collagen fibers are well-organized and intimately bound to one another, lamellar bone has greater rigidity and tensile strength and less elasticity than woven bone.
Cortical bone
Cortical bone is composed of dense compact bone, and its thickness depends on its location and mechanical requirements (Fig. 1.4). Cortices are thickest in regions exposed to large torsional forces, such as the central portion of the diaphysis, and thinnest where the transmission of torsional forces is smallest, as seen adjacent to articular surfaces, within vertebral bodies, and adjacent to the subperiosteal bone collar (SPBC), which borders the physis (3, 4).
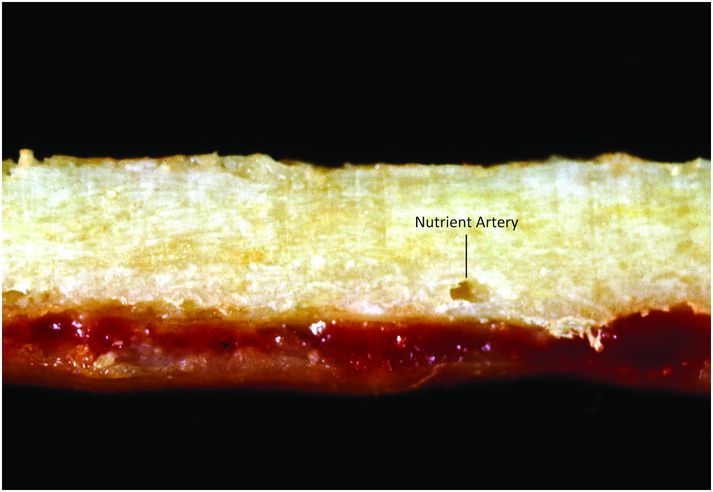
Figure 1.4 Cortical bone is dense and compact; note the foramen for the nutrient artery.
Mature cortical bone is composed of three different architectural lamellar patterns: circumferential, concentric, and interstitial (Fig. 1.5). The circumferential lamellae form outer and inner envelopes to the cortex and consist of several subperiosteal and endosteal layers that are oriented parallel to the long axis of the bone. They are the first cortical lamellae to be deposited, and in young individuals comprise almost the entire cortex. As mechanical stresses on the bone increase with age, many of the circumferential lamellae (except for several lamellae just beneath the periosteum and along the endosteum) are replaced by the concentric lamellae of the haversian systems. Haversian systems, or osteons, are initially created by osteoclastic resorption of the circumferential lamellae, and this process usually begins on the endosteal surface of the cortex, and less frequently on the periosteal surface. The accrual of layers of concentric bone over time reduces the diameter of the haversian canal so that in the end it is small and contains nutritional blood vessels and nerve twigs (Fig. 1.5). Together, these elements define a haversian or osteonal system.
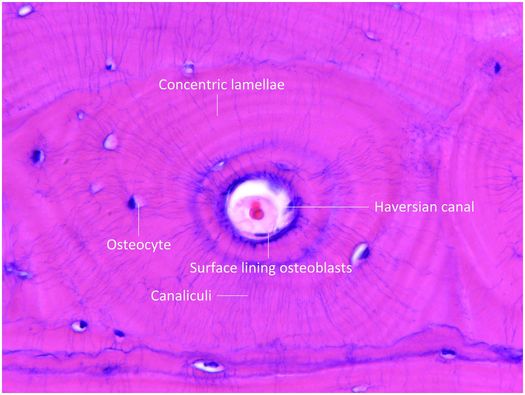
Figure 1.5 Histology of haversian system composed of the canal and its contents, concentric lamellae, osteocytes and the surface lining osteoblasts. The canaliculi containing osteocyte cell processes are prominent.
Mature haversian canals are long and cylindrical, range from 25 to 125 μm in diameter (average 50 μm), and are widest nearest the medullary cavity. They form an intricate, branching, spiraling, and interconnecting network that courses throughout the cortex. The number of haversian systems in a particular bone is variable and is determined by age, the amount of mechanical stress and weight the bone is subjected to over time, and other biologic and genetic factors.
Filling the spaces between the haversian systems is the interstitial bone. The interstitial lamellae represent the remnants of concentric lamellae of previously formed haversian systems that have become partially destroyed by osteoclastic activity. Interstitial lamellae are irregular, geometric-shaped units of lamellar bone that help “glue”, or anneal haversian systems to one another – this arrangement is important in maintaining cortical integrity. As the bone is subjected to varying forces and remodeling, newly formed haversian systems replace pre-existing interstitial lamellae and older haversian systems subsequently become newly created interstitial lamellae.
Cancellous bone
Cancellous bone is fenestrated, and is situated within the medullary cavity (Figs 1.6, 1.7). It consists of interconnecting plates and struts of trabecular bone, and its total surface area is very large, which facilitates remodeling, and the ability of the skeleton to respond rapidly to the metabolic demands of the body. Bone trabeculae are deposited according to lines of mechanical stress to provide support and distribute large weight-bearing forces along a variety of different pathways. Consequently, cancellous bone is most abundant in the weight-bearing ends of bones, such as the epiphyses and metaphyses of long bones and vertebral bodies, whereas it is present in only small quantities in the mid diaphysis of tubular bones.
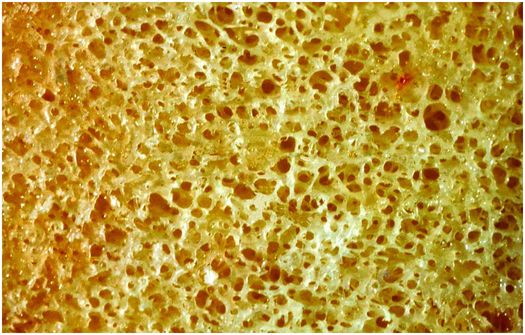
Figure 1.6 Cancellous bone is composed of interconnecting plates of trabeculae creating a large surface area for cell activity.

Figure 1.7 Trabeculae composed of the lamellae that are oriented in the same direction as the trabeculae.
Periosteum and perichondrium
The periosteum consists of a thin layer of tan-white connective tissue that covers the outer surface of all cortices. Where this layer of tissue overlies cartilage, such as that of the growth plate and adjacent immature epiphysis, it is known as the perichondrium. In children the periosteum is relatively loosely attached to the cortex, whereas in adults it is firmly anchored. The periosteum and perichondrium is constructed of an outer fibrous layer and an inner cellular or cambium (osteogenic) layer and these layers are most apparent during periods of rapid bone growth in children. The cambium layer is composed of fibroblasts, osteo- and chondro-progenitor cells, and developing osteoblasts and chondroblasts (Fig. 1.8). Generally, the number of progenitor cells present depends on the age of the child and the amount of bone cell activity in any particular region; they are especially numerous during periods of active bone formation. In contrast, in adults the periosteum appears largely as a fibrous layer that contains fibroblasts and broad collagen fibers that are continuous with those of the joint capsule, tendons, and muscle fascia. Collagen fibers of tendoligamentous structures pierce the periosteum and become anchored in the bone (Sharpey’s fibers).
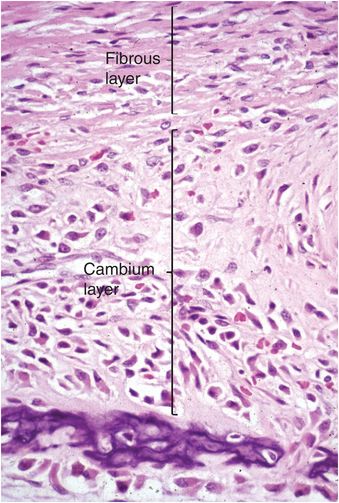
Figure 1.8 Fetal periosteum is composed of an outer fibrous layer containing collagen, and an inner cellular region known as the cambium (osteogenic) layer.
Vascular and nerve supply
Bones are very vascular and receive their blood supply from three main sources: (a) large nutrient arteries (one to two per bone); (b) metaphyseal and epiphyseal vessels; and (c) periosteal vessels. Nutrient arteries enter long bones in the diaphysis, traverse the cortex through foramina, and divide into ascending and descending branches within the medullary cavity. Smaller branch arteries, arterioles, capillaries, venules, and veins traverse the medullary cavity, nourish the fatty and hematopoietic marrow, and extend into haversian canals, where they supply the inner two-thirds of the cortex. At the ends of growing bones, small arteries give rise to a rich bed of capillary loops adjacent to the bases of the growth plates. The epiphyseal and metaphyseal vessels access bone through small apertures and provide blood flow to regions of the epiphysis and metaphysis in the mature skeleton and to the secondary centers of ossification during active enchondral ossification. The periosteal vessels are small and are believed to nourish the outer third of the cortex. The venous drainage system of bone is composed of medullary sinusoids that empty into a central venous sinus, which merges with nutrient veins.
Nonmyelinated nerves that are derived from the autonomic nervous system are the main source of innervation of bones, and their function is to control blood flow. Larger nerve branches are usually associated with arterial vessels, whereas small groups of fibers can be found adjacent to vessels in haversian systems. Nerves supplying the periosteum contain sensory elements and are responsible for the generation of bone pain.
Bone cells
The cells of bone are of different types and include the osteoblast lineage, osteoclast lineage, fibroblasts, adipocytes, smooth muscle cells found in vessel walls, endothelium, axonal processes, Schwann cells, and the hematopoietic elements. The cells responsible for the formation and remodeling of bone tissue are the osteoblast and osteoclast lineages.
Osteoblast lineage
Osteoprogenitor cells are derived from tissue-bound mesenchymal stem cells located in the peri-anlage tissue of the fetus, the periosteum, the haversian and volkman canals, and the medullary cavity. Osteoprogenitor cells are primitive committed mesenchymal cells that produce offspring that develop into osteoblasts. The process of osteoblast differentiation and maturation is complex and involves a variety of molecules and signaling pathways (5–14). Morphologically, the osteoprogenitor cells have the features of spindle cells, and do not have any distinguishing histologic characteristics.
Osteoblasts are responsible for the production, transport, and arrangement of most of the components of the organic bone matrix (osteoid). Importantly, they also regulate matrix mineralization, and use autocrine and paracrine mechanisms to influence the activity of neighboring bone cells. Osteoblasts have a lifespan that ranges from months to many years, and their metabolic state is reflected in their morphology. Osteoblasts actively synthesizing bone are polyhedral, have abundant cytoplasm that is in intimate contact with the bone-forming surface, and their nuclei are polarized away from the matrix surface (Fig. 1.9). As their synthetic activity diminishes, they become more attenuated and elongate (spindle-shaped) and remain as a cellular lining covering all bone surfaces (Fig. 1.10).
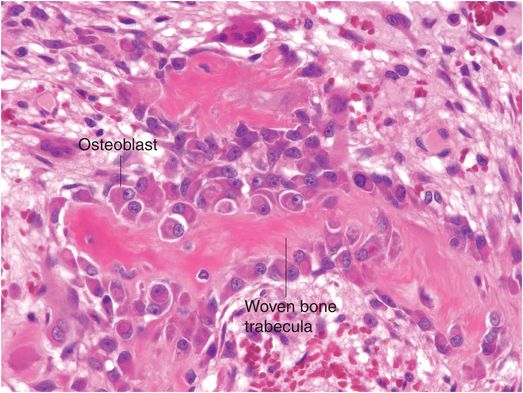
Figure 1.9 Metabolically active osteoblasts line woven bone trabeculae. The large osteoblasts have abundant cytoplasm and the nuclei tend to be oriented away from the bone-forming surface.
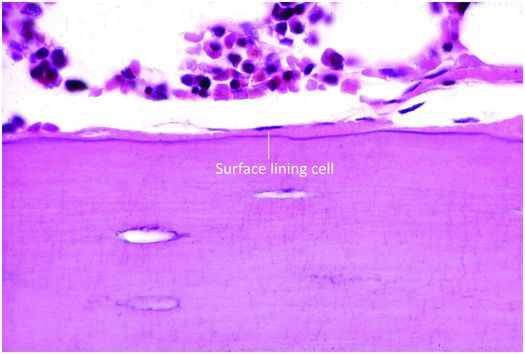
Figure 1.10 Quiescent osteoblasts, known as surface lining cells, covering trabeculae.
Osteoblasts enveloped by bone matrix are known as osteocytes (Fig. 1.5), and they are the most numerous cell type of the osteoblast lineage (15). The number, size, shape, and position of osteocytes vary according to the type of bone they inhabit. In woven bone, they are numerous, large, and plump, and are comparatively fewer in number, smaller, and more elongate in lamellar bone (Fig. 1.3). Osteocytes reside in lacunar spaces that house the cell body, nucleus, and surrounding scant cytoplasm. Osteocytes have numerous long and delicate cytoplasmic processes that extend beyond the lacuna, and traverse the matrix through small channels termed canaliculi (Fig. 1.5). This arrangement creates a very large surface area of contact between the osteocyte and the matrix and extracellular fluid that bathes each cell. Osteocyte cell processes connect to those of neighboring osteocytes and to surface osteoblasts via gap junctions. Gap junctions are specialized to facilitate the transfer of small molecules and biologically generated electrical potentials from one cell to another. In this manner, osteocytes communicate with one another and form a complex and integrated network throughout bone tissue.
The repertoire of biologic activity possessed by osteocytes helps them maintain bone tissue, and allows bone to be responsive to the mechanical and metabolic requirements of the body. As mechanosensory cells they translate mechanical forces into biologic activity (16–18). The detection of physical forces stimulates osteocytes to produce and release intercellular messengers that target surface lining cells, precursor cells, and osteoclasts (16–19). These cells, in turn, respond by remodeling the bone regionally as the structure and mass of the bone is altered according to the demands of the external physical environment. Osteocytes also generate and respond to microfluxes in ion concentrations and mediate the exchange of calcium and other ions between the bone matrix and extracellular fluid. In certain conditions, they may even be able to rapidly release calcium and phosphorus from the mineralized matrix by a process termed osteocytic osteolysis, and this manifests histologically as enlarged lacunar spaces (20). Additionally, osteocytes produce fibroblast growth factor-23, a hormone essential in the regulation of serum phosphorus as it modulates the reabsorption of this element in the renal tubule (17).
Osteoclasts
Osteoclasts are multinucleated cells responsible for the resorption of bone and cartilage. The matrix they break down must be mineralized for the process to be initiated and completed. Osteoclasts are mobile cells that have a lifespan of only several weeks. By the time they are recognizable by light microscopy they are fully differentiated and biologically active and reside within resorption pits (Howship’s lacunae) formed by their digestion of mineralized bone matrix (Fig. 1.11).
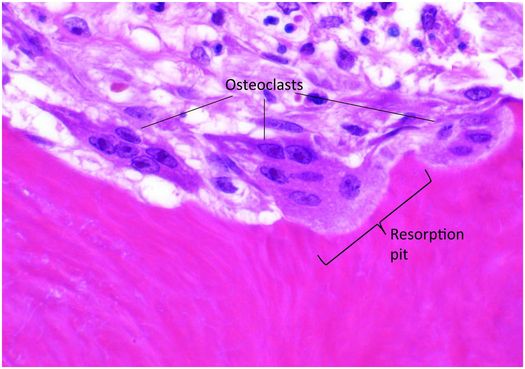
Figure 1.11 Three multinucleated osteoclasts reside in their respective resorption pits, which appear as scallops on the bone surface.
Osteoclasts are 40–100 μm in diameter and are polarized, with one portion of the cell membrane intimately attached to the bone and the remainder exposed to the extracellular fluid in its microenvironment. The cytoplasm in the vicinity of the resorbing surface is rich in lysosomes containing a variety of enzymes, and when their contents are released into the resorption pit, the actual process of bone digestion begins. Once osteoclast activity concludes and the cell moves to another targeted site, macrophages migrate into the base of the resorption pit and break down the organic remnants.
Osteoclasts are derived from mononuclear, hematopoietic progenitor cells of the granulocytic-macrophage colony-forming (GM-CFU) and macrophage colony-forming units (M-CFU) (21). The process of formation and activation of osteoclasts is controlled by a paracrine system that includes the receptor activator for nuclear factor κβ (RANK), RANK ligand (RANKL), and osteoprotegerin (OPG) (22–25).
RANK is a member of the tumor necrosis factor (TNF) family of receptors expressed mainly on cells of macrophage/monocytic lineage, such as preosteoclasts. When this receptor binds its specific ligand (RANKL), which is expressed by osteoblasts and marrow stromal cells through cell-to-cell contact, a series of signal cascades is activated and osteoclastogenesis is initiated. Another member of the TNF family of receptors that can block the actions of RANKL, is OPG, a soluble protein produced by a number of tissues, including bone, hematopoietic marrow cells, and immune cells. OPG inhibits osteoclastogenesis by acting as a decoy receptor that binds to RANKL, thus blocking the interaction of RANK with RANKL. The communication between bone cells and these molecules permits osteoblasts and stromal cells to control osteoclast development. This ensures the tight coupling of bone formation and resorption vital to the success of the skeletal system and provides a mechanism for a wide variety of biologic mediators (hormones, cytokines, and growth factors) to influence the homeostasis of bone tissue.
Skeletal growth and development: bone formation, growth, modeling, and remodeling
Beginning in the embryo until the stage that adult stature is attained, the bones of the body undergo a significant increase in size, refinement of shape, and enhancement of contour. Being rigid, bone cannot grow interstitially and only enlarges by the apposition of new bone on its surface. In contrast, cartilage has the capacity for both appositional and interstitial growth; it increases its substance and enlarges in all dimensions by adding new cells that elaborate freshly synthesized extracellular matrix internally, and on its surfaces. These characteristics of bone and cartilage form the foundation of the two major processes of formation and growth of the skeleton known as enchondral and intramembranous ossification. In enchondral ossification a cartilage model is replaced by bone and in intramembranous ossification bone tissue is formed directly by precursor cells residing in a membranous layer of fibrous tissue.
Enchondral ossification and growth plate cartilage
The increase in length of tubular bones in embryos and prepubertal children occurs as growing cartilage is replaced by bone, with the majority of the growth derived from the cartilage anlage (cartilage model of the future bone) and growth plate (physis). The cartilage anlage originally develops in the early stages of embryogenesis from mesenchymal cells that form cellular condensations at the sites of future bones (26, 27). These mesenchymal cells differentiate into chondrocytes that produce a cartilage model or anlage of the forthcoming bone (Fig. 1.12). This process commences at a specific time for individual bones, and the temporal sequence of anlage formation is the same in all humans. Surrounding the newly formed cartilage anlage are several layers of mesenchymal cells that form the perichondrium, which gives rise to progenitor cells that differentiate into chondrocytes (Fig. 1.12). Subsequently, a specific portion of the perichondrium transforms into periosteum to initiate ossification. This process first occurs in the mid portion of the cartilaginous shaft where mesenchymal stem cells in the perichondrium begin to produce a layer of osteoblasts that deposit a collar of woven mineralized bone on the surface of the cartilage model. This anatomic site is known as the primary center of ossification (Fig. 1.13).
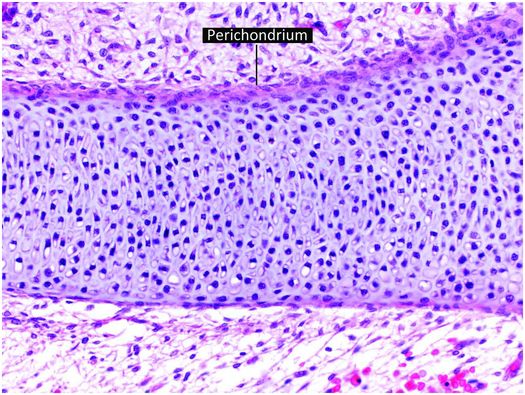
Figure 1.12 Cartilage anlage composed of hyaline cartilage. The perichondrium covers the surface of the structure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree